September 1, 2018 (Vol. 38, No. 15)
PendoTECH’s Durability and Accuracy Key to Using Single-Use Sensors for Extended Periods of Time and for Sensitive Applications
The growth of continuous processing in the bioprocess industry is driving demand for sensors that can be used reliably for extended periods of time with no degradation in sensor performance. This push toward continuous manufacturing, coupled with the benefits of single-use technology, creates demand for single-use sensors that have proven performance during long-term use. The following article details extensive lab testing conducted by PendoTECH to demonstrate the reliability and performance of PendoTECH Single-Use Pressure Sensors™ over time. This data demonstrates that PendoTECH sensors are durable enough to meet the demands of continuous processing with no loss in performance compared to more traditional, nondisposable technologies.
In addition to being robust enough for continuous processing, PendoTECH single-use pressure sensors are accurate enough for processes requiring highly precise measurements, including leak testing. It is critical to ensure the integrity of disposable bags prior to use in processing. PressureMAT™ HR monitors, along with single-use pressure sensors, are a cost-effective and reliable means of performing leak testing without compromising the sterility of a disposable system.
Requirements for Use in Continuous Processing
For single-use sensors to be viable in a commercial continuous processing facility they must be made of highly durable materials and be available in smaller sizes than sensors traditionally used in large batch processes. All product contact components used in PendoTECH single-use pressure sensors are designed and have been tested to withstand continuous use of up to 90 days. Any use of PendoTECH single-use sensors for greater than 90 days should be evaluated by the end user before incorporating into their process.
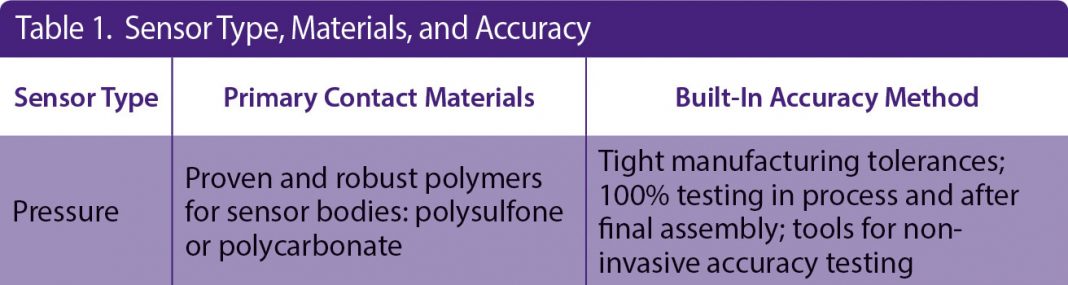
Another challenge of incorporating single-use sensors into a GMP process is the closed nature of the bioprocess system and associated single-use manifold. Generally, this manifold arrives presterilized, and thus calibration cannot be done at time of use without compromising the sterility of the system. PendoTECH has developed a solution for each single-use sensor, and associated monitor, resulting in the claim of “no calibration required” at the point of use. The methods used to make this claim for single-use pressure sensors are shown in Table 1 under “Built-In Accuracy Method.”
In addition to examining the impact of time and type of exposure of the sensor materials, during a continuous process of up to 90 days, the susceptibility of the sensor to measurement drift/change in calibration was examined. Finally, during continuous processing, it is imperative that all data from the sensors be transmitted in real time to higher-level control systems and to data historians. The method in which PendoTECH monitors communicate with higher-level control systems will also be discussed.
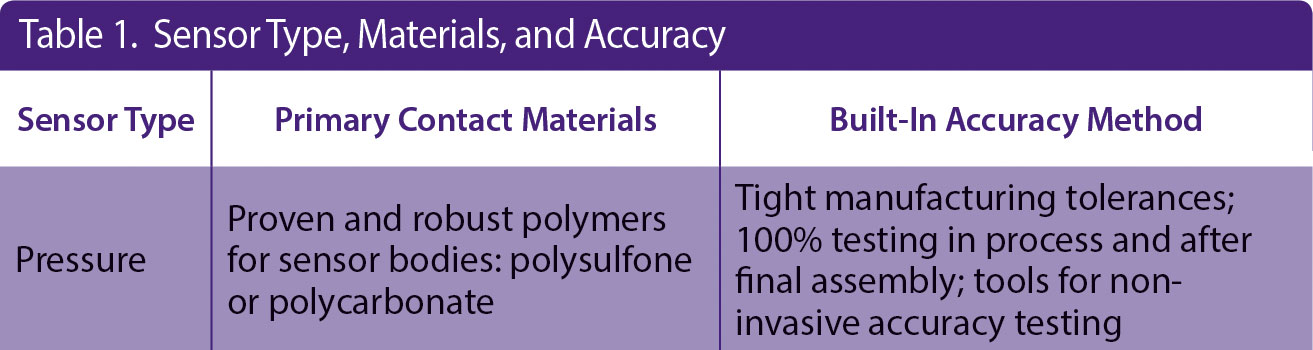
Table 1. Sensor Type, Materials, and Accuracy
Single-Use Sensor: Materials of Construction, Sizes, and Technology
For use in extended continuous processes, single-use sensors must be designed for single use without sacrificing performance and lifetime compared to traditional stainless-steel instruments. The materials used in PendoTECH single-use pressure sensors are well-characterized and highly durable polymers commonly found in other biopharmaceutical and medical device products that require long life cycles.
Because of the lower flow rates and smaller working volumes found in some continuous processes, relative to traditional batch operations, small ID tubing and associated sensors are desired to increase linear velocity inside the tubing, promoting uniform flow and preventing settling from occurring. To meet these requirements, PendoTECH single-use pressure sensors are available with 1/8-inch hosebarb specifically designed for flow rates less than 15 mL/min. Conversely, PendoTECH single-use pressure sensors are also available with inlet/outlet up to 1-inch hosebarb and 1.5-inch TC to accommodate larger scale processes. The complete range of sizes is shown in Figure 1.
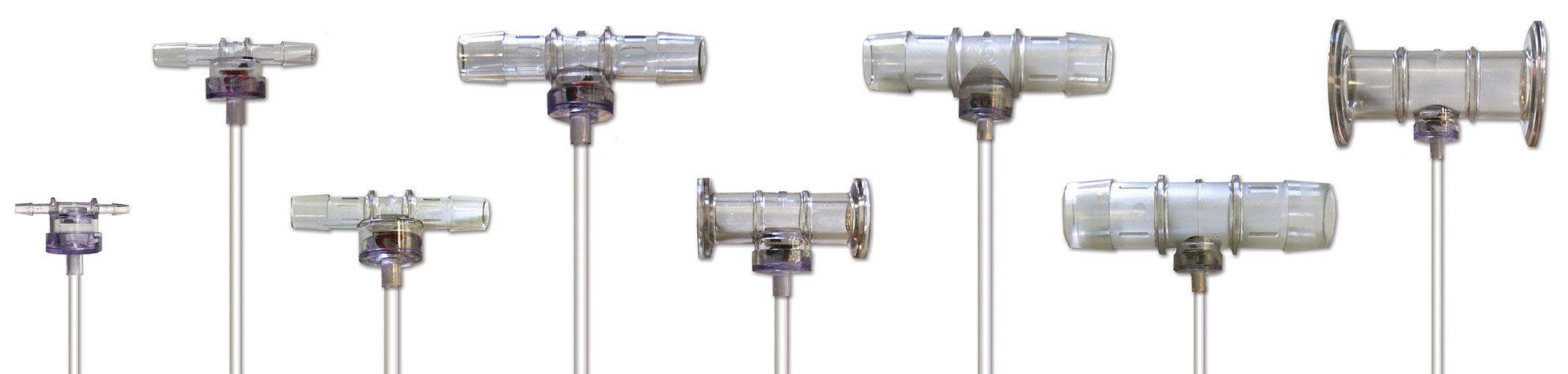
Figure 1. PendoTECH’s Single-Use Pressure Sensors measure static and dynamic pressure of gases and liquids in biopharmaceutical processes. As this photo indicates, these sensors are available in a range of sizes.
Performance and Results
The growth of continuous processing coupled with the benefits of single-use technology creates demand for single-use sensors that have proven performance during extended use. To demonstrate the performance of PendoTECH single-use sensors over time, lab studies on PendoTECH single-use pressure sensors were carried out, demonstrating that the sensors maintain accuracy during periods of prolonged continuous use.
PendoTECH also conducted studies to demonstrate the accuracy of its PressureMAT-SHR™ model. This “high-resolution” monitor is specifically designed for very-low-pressure measurements. The full test procedure and results are available by contacting PendoTECH, but an overview of the test is presented at the end of this article.
Integration to Higher-Level Process Control Systems
Single-use sensors and their associated monitors should have built-in communication protocols allowing for data to be transmitted, in real time, to higher-level control systems and data historians. Analog signals, such as 4–20 mA, remain popular due to their simplicity. But digital communication protocols are becoming increasingly popular as production facilities continue to grow more sophisticated.
Figure 2 illustrates a generic plant architecture diagram. The sensors and monitors occupy the boxes to the left, while higher-level control systems are represented by the boxes on the right half of the diagram. Monitors that have built-in transmission functions can be seamlessly integrated into existing plant architecture via analog or digital output signals.
Monitors, such as those offered by PendoTECH, have communication options for integration to existing plant architecture. They also have verification tools to test their performance independent of the sensor. This feature can be used to test the communication chain from the monitor.
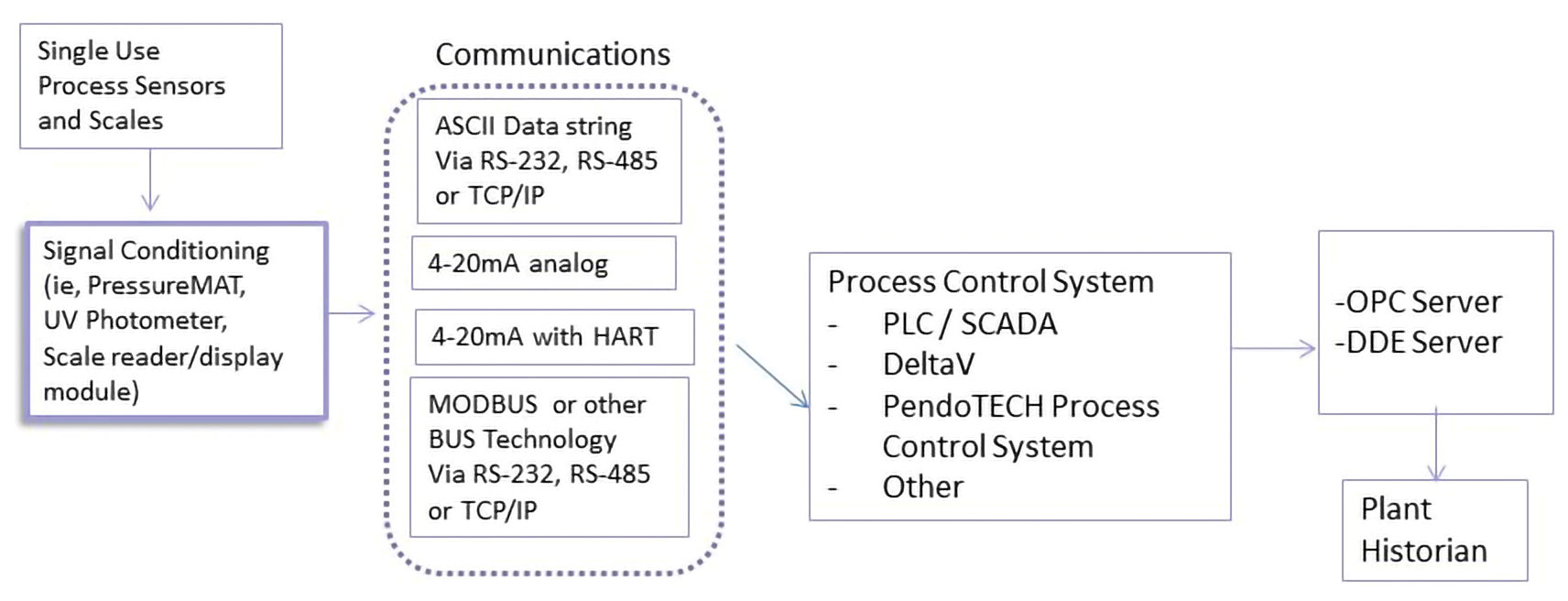
Figure 2. This diagram shows sensors and monitors may be integrated into an existing plant architecture if transmission and communications functions are available.
Conclusions
In summary, PendoTECH single-use sensors are robust enough to be considered for qualification in GMP processes. Sensors incorporated into a continuous processing facility must be evaluated based on process-specific user requirements—such as required sensor lifetime, compatibility, and process accuracy requirements. PendoTECH single-use sensors are accurate enough for sensitive applications, such as leak testing, and can be read locally as well as integrated into higher-level systems.
Overview of PendoTECH’s Performance Tests
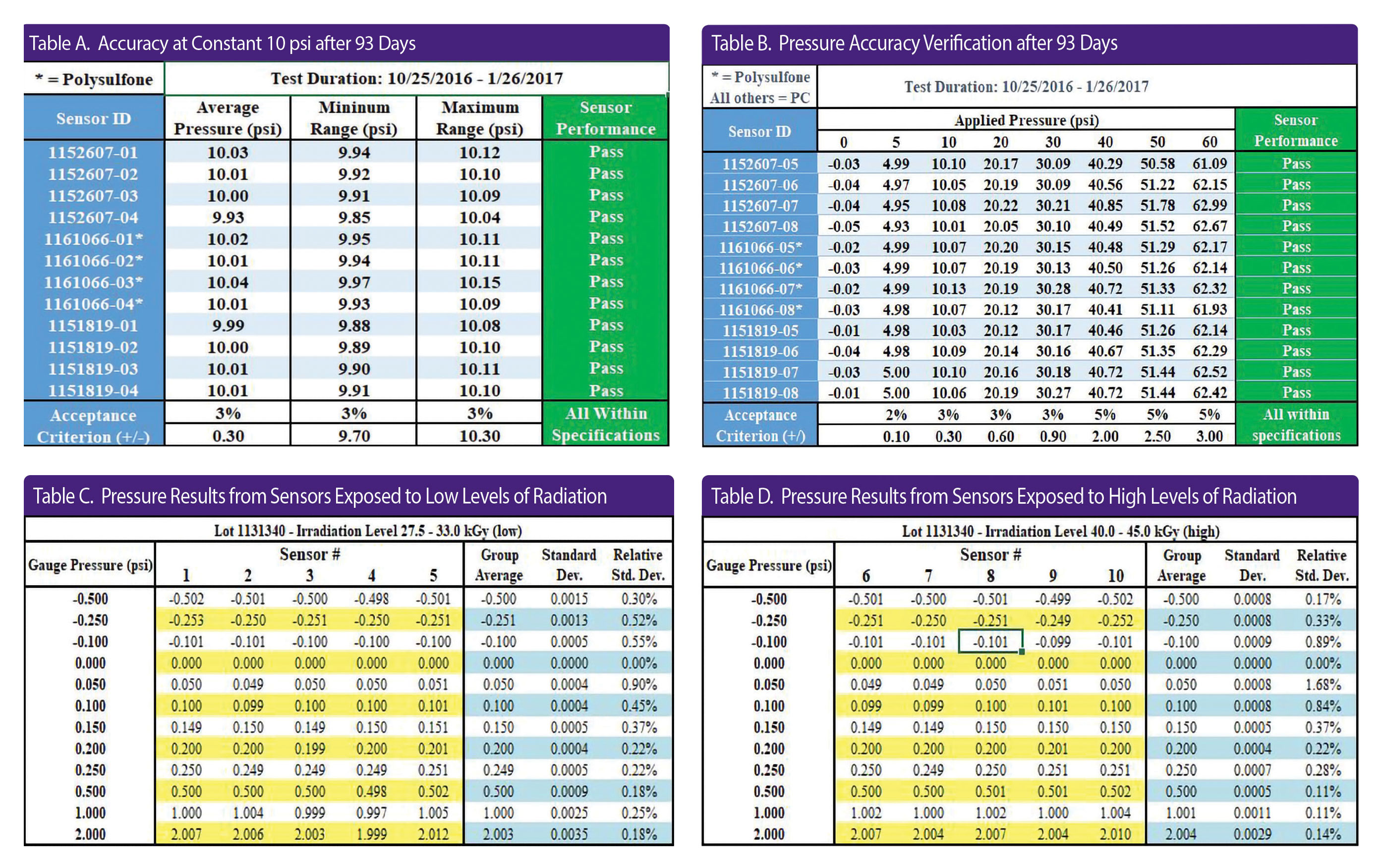
To demonstrate the long-term accuracy of PendoTECH single-use pressure sensors, a randomly selected group of sensors representing three lots of product were exposed to a constant pressure of 10 psi for 93 days. Tables A & B present information gathered during testing as well as post-experiment sensor verification, demonstrating sensor performance versus a calibrated gauge. No tare of the sensors was conducted post-exposure.
After 93 days exposure to 10 psi, the pressure sensors remained accurate. Overall, the test results clearly show the PendoTECH single-use pressure sensors remain well within their stated accuracy specification over the life of the experiment.
Use in System Leak Testing
Some applications, such as leak testing of disposable bags, require accurate pressure readings to at least three decimal places. It is highly advantageous to be able to conduct these tests with a single-use pressure sensor, preinstalled on the bag/manifold, so that sterility is not compromised during this test.
To address this requirement, PendoTECH developed a modified version of its PressureMAT™ (PMAT) monitor for single-use sensors. This version is the “high-resolution” (HR) model. In contrast to the standard monitor that can read up to 75 psi, HR models have been modified and optimized for low-pressure measurements to 7.5 psi (~0.5 bar). This is ideal for low-pressure applications, and the zero-offset stability (ZOS) is reduced to
PendoTECH conducted tests to demonstrate the effectiveness of the PMAT-SHR, and single-use pressure sensors, to measure extremely low pressures after gamma irradiation.
Testing Process
In this experiment, 30 sensors were randomly selected from three separate lots and exposed to two different levels of gamma irradiation: 27.5–33 kGy (low) and 40–45 kGy (high).
Using a low-pressure test pump in conjunction with the Crystal XP2i (from Ametek) high-accuracy pressure test gauge, the sensors were subjected to vacuum at −0.50, −0.25, and −0.10 psi, and then pressurized to 0.05, 0.10, 0.15, 0.20, 0.25, 0.50, 1.00, and 2.00 psi. The pressure displayed on the PMAT-SHR pressure monitor was recorded at each pressure or vacuum level, and results were compared with that from the Crystal gauge.
Results
Tables C & D report the testing results after applying a known pressure/vacuum to each sensor from one lot exposed to both irradiation levels. The averages, standard deviations, and relative standard deviations were calculated and reported for sensors grouped by lot and irradiation level. These results show that group averages, measured to three decimal places, are nearly identical to the applied pressure. The accuracy specification of the sensors is ±2% of reading plus the 0.003 psi ZOS.
Looking at the data in both tables, all the data points on the 10 sensors tested meet that accuracy specification. The statistics also demonstrate the precision of the sensor/PMAT-SHR combination.
For potential use in a pressure-hold/leak-test application, the contribution of the 0.003 psi ZOS can be calculated by the following example. Populate the following formula based on testing a single-use assembly with an approximate bag volume of 1,000 L for converting pressure hold to leak rate from:
Rate =(ΔP × Vsystem)/(Test time × Patm)
=(±0.003 psi × 1,000 L)/(10 min × 14.7 psi)
= ±0.020 L/min
During a potential test, you could expect to see a potential fluctuation of ±0.003 psi from the PMAT-HR corresponding to a value of ±20 mL/min during the test that would not be interpreted as a leak. This rate value would go up or down proportionately with larger or smaller containers. This ZOS factor would need to be considered for any test design. And, relative to the single-use systems having a constant volume (V), a settling time should be considered before the final tare to achieve rigid position of the somewhat flexible components.
Conclusion
Leak-testing/pressure-decay on single-use systems with air is feasible by applying the ideal gas law as long as a pressure-sensing device can measure to the accuracy required for a low-pressure system, such as in single-use bioreactors and storage bags. The data presented here indicate that using the PMAT-HR monitor with PendoTECH single-use pressure sensors provides excellent experimental results for high-accuracy pressure measurement in the range of −0.500 to 2.000 psi.
Furthermore, the data show that two typical levels of gamma irradiation make no significant difference in the accuracy or consistency of results. The increased resolution of the PMAT-HR by 10-fold over the standard monitor reduces the error of a low-pressure process measurement and improves its capability for leak testing in single-use sensors.
Tables A-D
Continuous Bioprocessing: Brave Old Challenges with Bold New Technologies
Visions of a Continuous Bioprocess Future
Continuous Processing’s Benefits within Biopharma’s Reach
Continuous Low-pH Virus Inactivation: Challenges and Practical Solutions
Vero Perfusion, Packed-Bed Vessels Intensify Vaccine Production
A Swiss Army Knife for Modern Biomanufacturing
Next-Generation Processing a Multidisciplinary Pursuit
Next-Generation Bioprocess Techniques