September 1, 2018 (Vol. 38, No. 15)
Andreas Castan Ph.D. Bioprocess R&D Scientists GE Healthcare Life Sciences
Helena Ohrvik Ph.D. Bioprocess R&D Scientists GE Healthcare Life Sciences
Daniel Nelson Senior Product Marketing Leader, Upstream GE Healthcare Life Sciences
GE Healthcare Mapping Roads to the Future
Large biotechs, emerging biotechs, and CMOs alike are increasing investment in novel bioprocessing approaches to achieve success in the rapidly evolving biotherapeutic market. Addressing diverse molecule pipelines and uncertain demand projections that depend on clinical outcomes requires that bioprocessing approaches increase manufacturing supply chain flexibility and utilization.
For example, novel buffer management and semicontinuous approaches are becoming commonplace as they represent powerful levers to increase facility utilization—a key driver of cost.
With many bioprocessing approaches to choose from, drug manufacturers should select a path based on defined outcomes that are aligned with their specific molecule pipeline and/or current manufacturing capabilities. To illustrate this, consider how decisions will be affected given the following pipelines: complex molecules (bispecific antibodies, enzymes, or viral vectors), high volume demand (e.g., large populations and/or large dose) therapies, or biosimilars (cost pressures driving down CAPEX and, potentially, cost of goods sold, or COGS).
How would these pipelines affect global supply chain and process considerations? How do their needs for flexibility, speed, and costs differ? This article will attempt to address these questions, because understanding available options and how to best deploy them in the right circumstances will be crucial for successful outcomes.
Process Productivity
Increasing process productivity is one of the most effective ways to improve facility utilization, which is largely synonymous with flexibility. For example, high-yielding upstream processes can produce the same amount of drug product in shorter periods of time or reduce footprint by being implemented in smaller bioreactors.
Additional flexibility, in this instance, occurs because the same pilot plant setup can be implemented at production scale, thereby reducing scale-up activities and enabling a scale-out mentality.
Furthermore, it is easier to transfer smaller processes to new geographies, which is necessary to establish and maintain a global manufacturing network—a key for establishing a global presence.
Some approaches, however, can adversely affect flexibility and require greater analysis in context of the overall business strategy. For example, while operating in upstream perfusion mode, the process times are prolonged, which can have considerable impact on a facility’s flexibility. There are some preliminary studies1–3 indicating that the impact of a perfusion process on the product’s COGS is strongly dependent on both the total yearly production output, or plant output, and the subsequent downstream process. Therefore, a complete business analysis and proposal for a continuous operation up- and downstream needs to be compiled.
Next-Generation Processes
So, what are next-generation processes? Next-generation processes overarch the whole manufacturing chain from cell to molecule, upstream to downstream. They include semicontinuous and continuous approaches, focus on operational excellence and the implementation of disruptive technologies up- and downstream, and encompass supplier partnerships. Here, we will center the discussion around two large concepts:
* Continuous manufacturing: Continuous unit operations with high volumetric productivities (Table 1)
* Process intensification for eliminating existing bottlenecks: often refers to the shortening of process times or reducing non-value-added process steps. In upstream processing, this can be exemplified by intensified seed-train (Figure 1) or intensified production bioreactor operations (Figure 2). In downstream processing, it can be intensified capture, inline virus inactivation, flow through polishing, inline virus filtration, or single-pass filtration.
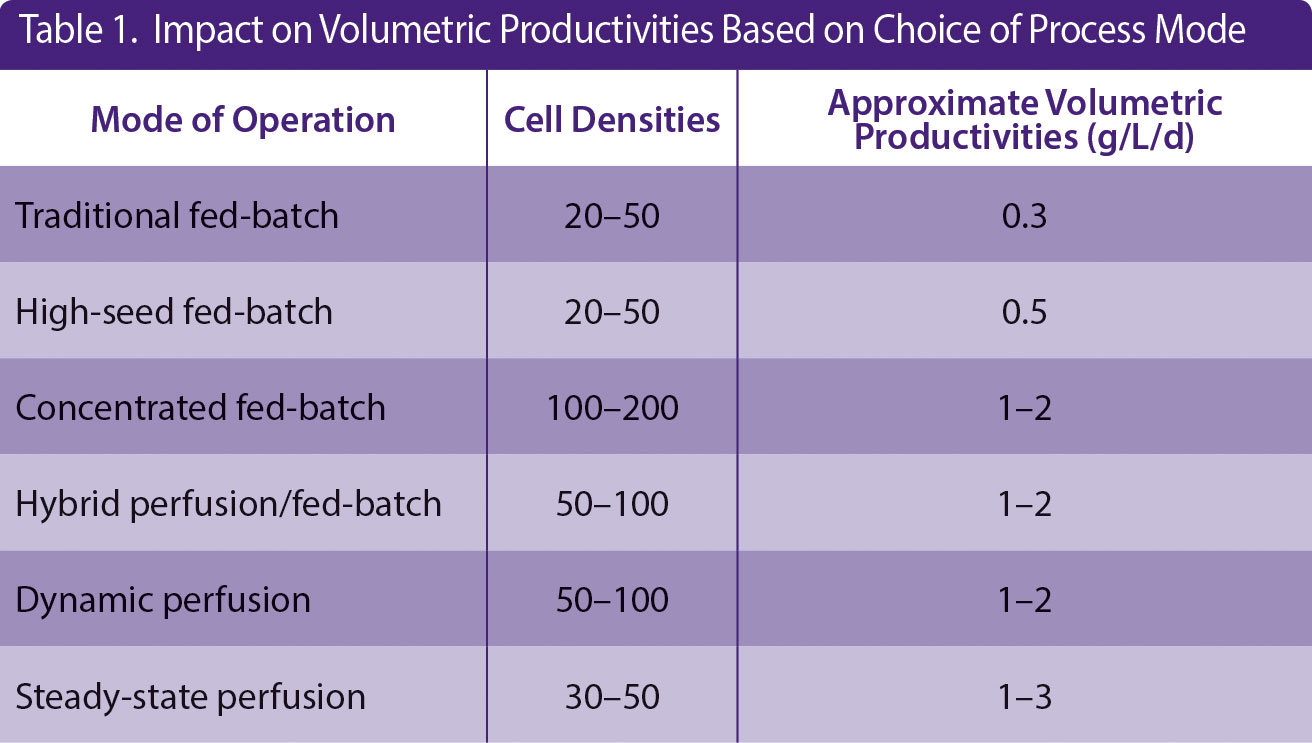
Table 1. Impact on Volumetric Productivities Based on Choice of Process Mode
While not covered in this article, it is important to note that success in both categories will be amplified by complimentary advancements in automation, e.g., digitalization, inline analytics, sensors, and inline process control. Other strategies underpinning successful deployment include raw material controls, supply chain security, supplier partnership, and single-use technology.
Today’s processes are often a hybrid of continuous and batch processes. Continuous upstream processes have been long established, and therefore represent lower risk for GMP implementation. This is in contrast with downstream operations, where regulatory concerns and understanding of critical process parameters for adequate control remain to be understood.
We anticipate more adoption of continuous upstream approaches to take advantage of the opportunity to create a greater impact in short-term development. The remainder of this article will focus on these approaches.
The seed train presents several process-intensification opportunities. One possibility is to increase the cell density (from 20 to 100 E6 cells per mL) and the volume (from 1 to 500 mL) during cryopreservation, called high-density cell banking. Another is to use perfusion in the seed bioreactors to eliminate processing time from subsequent steps. An application of both approaches for seed-train intensification is shown in Figure 1.4
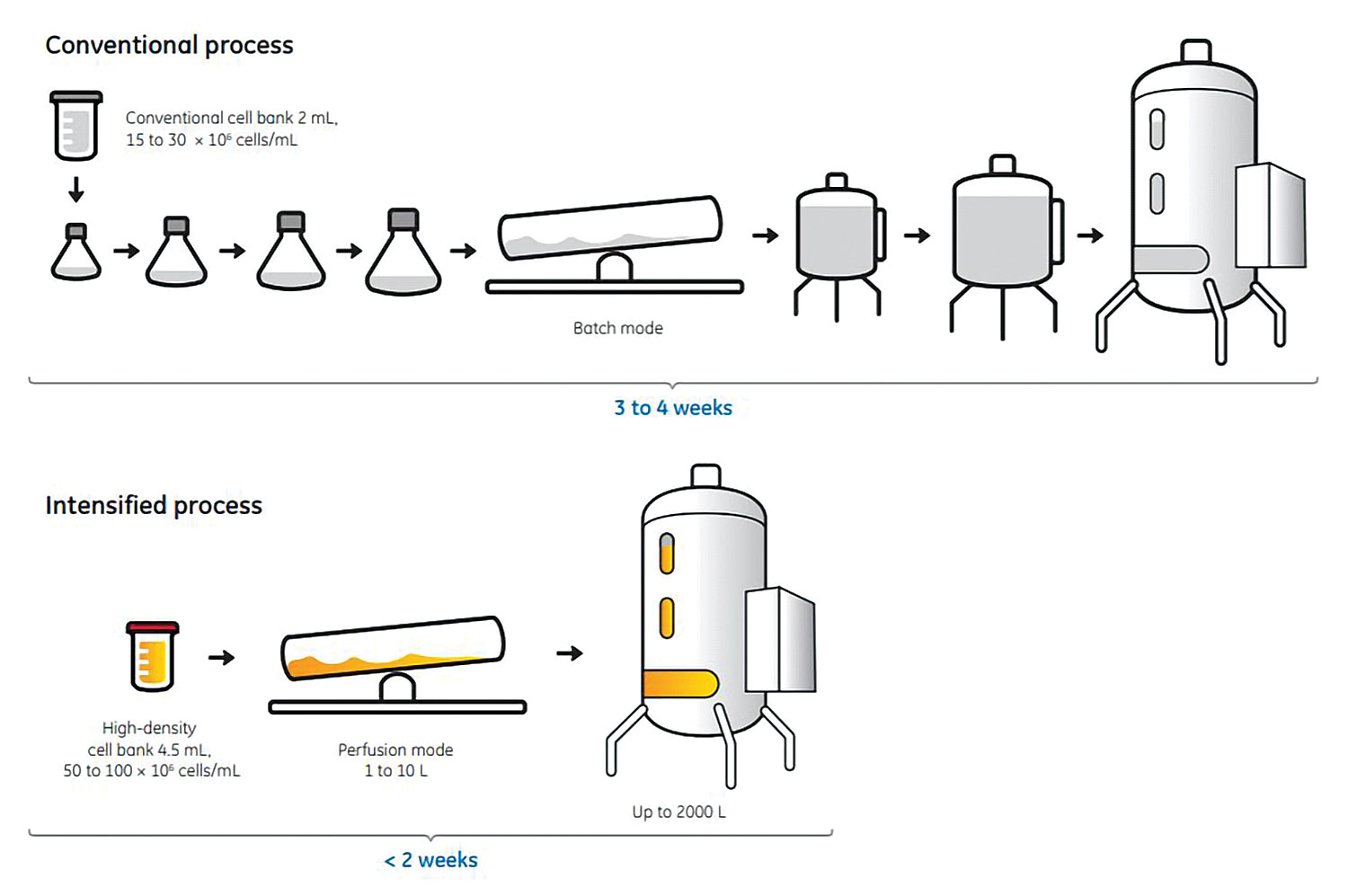
Figure 1. Vials from a high-density cell bank can be used for direct inoculation of a small bioreactor culture, eliminating the need for shake flask operations in the immediate post-thaw expansion phase. The use of perfusion in the bioreactor stage allows cells to stay in exponential growth phase throughout the entire culture, for high final viable cell density and cell viability. By such a procedure, an N-1 culture at 10-L scale can be used to seed a production culture with a final volume of 2000 L.
Several strategies may also be applied to production bioreactors for improving cell culture outcomes. Inoculating at a higher density (through perfusion in the N-1 step, e.g., resulting in inoculation densities of 10 E6 cells/mL instead of traditionally 0.5 E6 cells/mL), can shorten the time in the production bioreactor by about five days.5 Other modes of operation include intensified fed-batch, steady-state perfusion, dynamic perfusion, hybrid perfusion/fed-batch. An illustration of some of these modes of operation is given in Figure 2, and the impact is on the volumetric productivity is given in Table 1.
Perfusion mode presents the highest potential to increase volumetric productivities. However, only a small percentage of worldwide bioreactors are operated this way today. One reason for this is that many parameters need to be optimized, e.g., medium composition, steady-state cell density, strategies for cell separation, bleed rate, process automation, cell line stability, and product quality attributes, resulting in long process development time.
Table 2 gives an overview of what to consider for these parameters. Taken together, the perfusion parameters with the most impact on process economy and productivities are cell line productivity and the medium composition required to support high productivity and low media consumption.
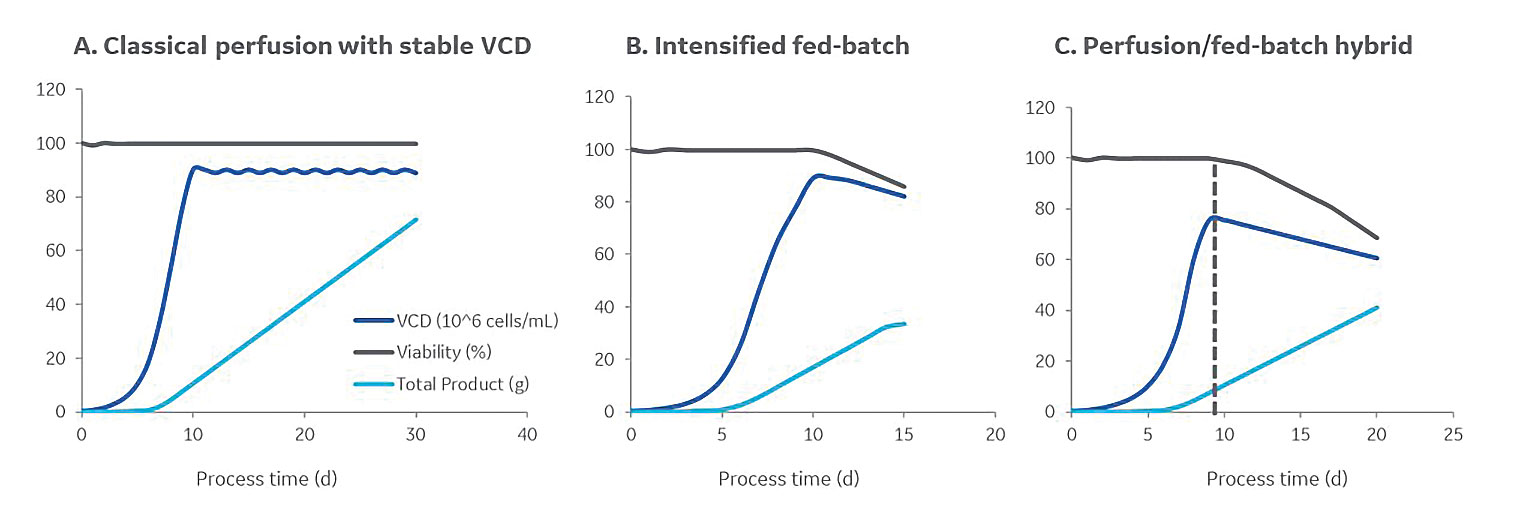
Figure 2. Several strategies may be applied to production bioreactors for improving cell culture outcomes. Alternatives include (A) classical perfusion, (B) intensified fed-batch, and (C) perfusion/fed-batch hybrid modes. The mode with the highest potential to increase volumetric productivity is perfusion seed culture. This mode, however, is relatively uncommon.
Furthermore, studies from mAb processes show how the perfusion rate and media composition have the potential to be used as tuning forks for product quality.6 Through the growth of the biosimilar market, a need has emerged for better and tighter control of product quality.
Consistent quality (or more stringent quality) has traditionally been demonstrated for the production of unstable proteins, such as coagulation factors in perfusion mode. Production at steady state has the promise of a more consistent product quality.
Biopharmaceutical companies adding more molecules to a facility, or facing increased volumetric demands, may choose to increase productivity and throughput using their existing infrastructure. For example, cell culture suites can be modified for concentrated fed-batch (CFB) operations using existing bioreactor platforms.

Table 2. Key Points to Consider in a Perfusion Process
However, for new classes of biologics and when designing new platform processes and creating a global manufacturing network, perfusion may be the better option. To determine which approach fits the pipeline and facility, it is important to analyze both current and future bottlenecks in the manufacturing plant, and how these can be mitigated, e.g., medium and buffer handling, inline buffer solutions, and raw materials.
Single-use technology is an important facilitator for these intensified processes. To unlock the potential of single-use technology, a partnership between suppliers and users is recommended. Already, today, many companies have engineers and technicians working directly with single-use vendors. This emphasizes the importance of partnership between suppliers and companies. In Table 3, we have summarized potential bottlenecks for the development of next-generation processes and outlined and discussed mitigation strategies to navigate in this jungle of possible solutions.
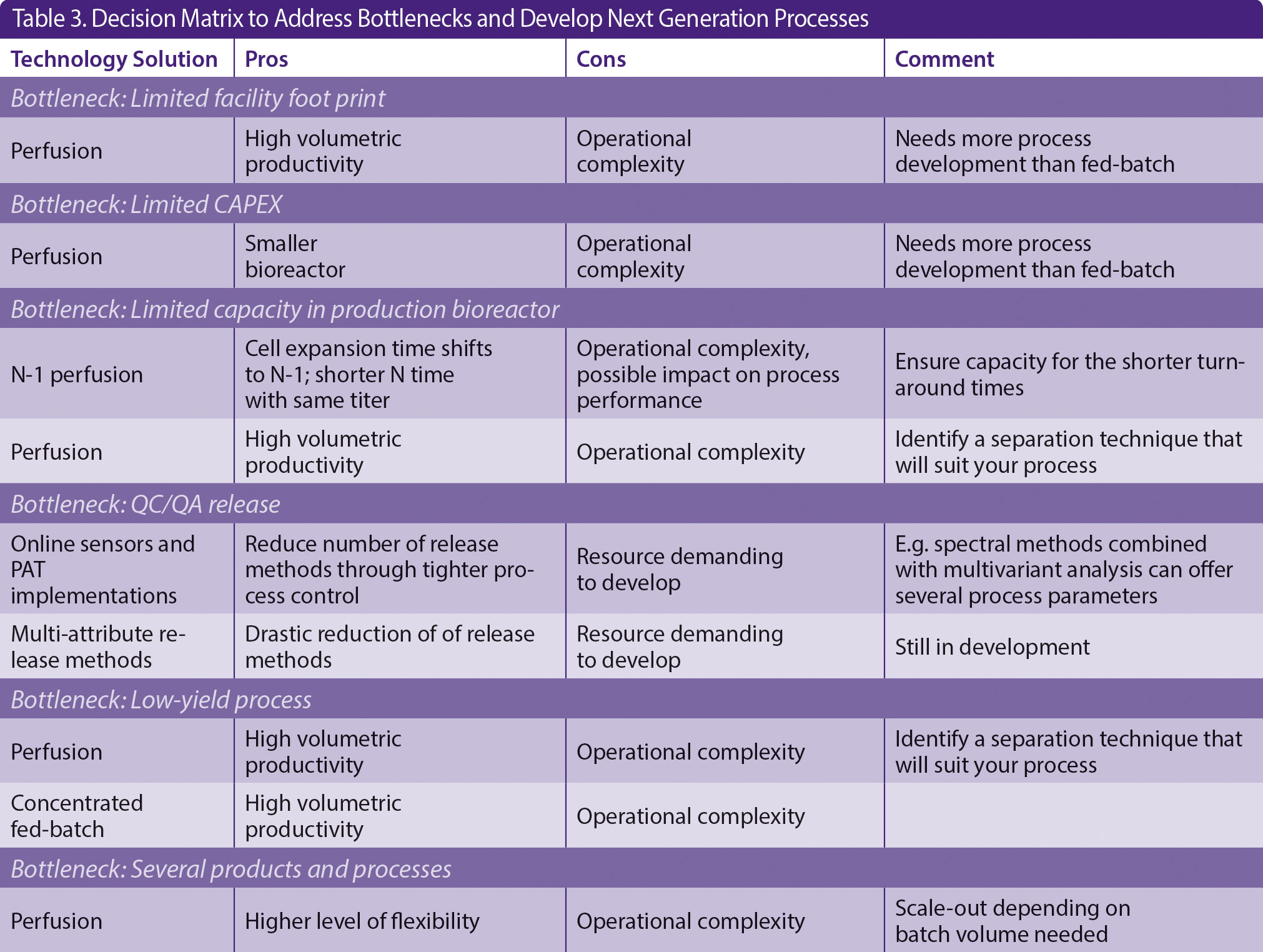
Table 3. Decision Matrix to Address Bottlenecks and Develop Next Generation Processes
Conclusion
When does it make sense to intensify your process and/or deploy continuous steps? With your molecule pipeline and facility/business strategy understood, we propose a situational analysis with respect to:
1. The mode of operation that is suitable for your plant (see Table 1)
2. The bottlenecks and key parameters for your processes, but also the critical product quality attributes
3. The business drivers for each mode (time, costs, pro/cons)
The choices may appear complicated, but here we have presented considerations that will aid in determining the right process and facility decisions when transitioning into next-generation bioprocessing approaches. Productivity and flexibility increases can be achieved when the right tools for single-use technology, hardware, automation, process design, and cell line and media development are combined.
For many, it is helpful to engage a knowledgeable partner to discuss and explore the range of options. A strategic partner that understands the process application and challenges of successfully implementing next-generation bioprocessing and has a successful track record of customer collaborations, can facilitate finding the optimal solution to fit their situation.
Andreas Castan, Ph.D., and Helena Öhrvik, Ph.D., are bioprocess R&D scientists, and Daniel Nelson is senior product marketing leader, upstream, at GE Healthcare Life Sciences.
1. Pollock J. et al., Fed-batch and perfusion culture processes: economic, environmental, and operational feasibility under uncertainty, Biotechnol. Bioeng. 110, 206–219 (2013).
2. Pollock J. et al., Optimising the design and operation of semi-continuous affinity chromatography for clinical and commercial manufacture, J. Chromatogr. A 1284, 17–27 (2013).
3. Walther J. et al., The business impact of an integrated continuous biomanufacturing platform for recombinant protein production, J. Biotechnol. 213, 3–12 (2015).
4. GE Healthcare, Application Note, “One-Step Seed Culture Expansion from One Vial of High-Density Cell Bank to 2000 L Production Bioreactor,” 29160932, Edition AB (2016).
5. Yang W. C. et al. Perfusion seed cultures improve biopharmaceutical fed-batch production capacity and product quality. Biotechnol. Prog. 30(3),
616–625 (2014).
6. Walther J. et al., Perfusion Cell Culture Decreases Process and Product Heterogeneity in a Head-to-Head Comparison with Fed-Batch, J. Biotechnol., doi.org/10.1002/biot.201700733 (2018).
Continuous Bioprocessing: Brave Old Challenges with Bold New Technologies
Visions of a Continuous Bioprocess Future
Continuous Processing’s Benefits within Biopharma’s Reach
Continuous Low-pH Virus Inactivation: Challenges and Practical Solutions
Vero Perfusion, Packed-Bed Vessels Intensify Vaccine Production
Single-Use Pressure Sensors for Continuous Processing and Leak Testing
A Swiss Army Knife for Modern Biomanufacturing
Next-Generation Processing a Multidisciplinary Pursuit



