September 1, 2018 (Vol. 38, No. 15)
Mark Schofield Ph.D. Senior R&D Manager Pall Biotech
David M. Johnson Global Product Manager, Chromatography Hardware Pall Life Sciences
Pall Biotech Describes an Automated and Semicontinous Multivessel System
Continuous processing can revolutionize biomanufacturing. Equipment size can be reduced, yet more product produced through increased cycling. Hold-tanks can be minimized or eliminated, allowing for near one-piece flow of the product. This in turn facilitates a much faster overall purification which may be highly desirable to produce labile products such as enzymes and clotting factors.
Product quality may also be improved through the increased automation and cycling that has to be embraced to operate continuously. Thus, continuous processing is predicted to reduce cost and improve quality, which benefits both patients and the industry.
The adoption of continuous processing introduces some technical challenges. Virus safety has not been completely addressed; however, the regulatory authorities take the stance: “implement an updated design of viral safety evaluation for the processing mode.”1 For monoclonal antibodies (mAbs) virus clearance is normally achieved through an orthogonal approach, including chromatography, low-pH inactivation, and filtration. Adopting continuous processing undoubtedly has an impact on each of these steps.
Perhaps the greatest challenge lies in adapting the low-pH virus inactivation (VI) step to continuous operation. For current batch processes this step is routinely performed manually. But the move to continuous requires the process to be automated; it must also accommodate a change in the cadence of the Protein A capture process which will produce multiple elutions per hour over a time course that could be up to several months. This has led to the development of some different strategies, including a continuous plug-flow reactor and packed column–based low pH hold to process and attenuate these elution volumes.
Continuous Reactor
A continuous or plug-flow reactor would initially appear to be the ideal way of performing low-pH VI. Elutions can be adjusted inline, directly to the hold pH, as they leave the Protein A column. From here the elutions are passed through a tubular reactor that ensures that the product is retained for the inactivation time.
The advantages of this approach are that elutions are processed continuously and could provide a solution as close as possible to a continuous flow.2,3 However, there are a number of technical challenges:
1. Axial dispersion in a tube. The flow toward the center of a channel is faster than the flow at the outside of the channel. This has been addressed by using a coiled path geometry4 that ensures minimal forward and reverse dispersion during transit through the attenuator. The scalability of this is far from simple, as axial dispersion is an exponential function of the internal diameter of the attenuator. Thus, the plug-flow behavior is likely to require validation at scale.
2. Effect of diffusion. It is unclear what effect discontinuous elutions and pump pulsing might have on the required hold time and how to validate for this.
3. Elutions come ahead of the elution buffer and vary in protein concentration across the peak. This makes titration to the inactivation pH challenging given a continuously changing elution composition.
4. pH probes have some hysteresis, so how can it be ascertained that the complete elution has achieved the inactivation pH? In plug-flow reactors the pH reading can be used only as a monitor, rather than as part of a controlled titration.
5. With this approach there is no pooling at the hold pH, so given the varying elution composition an acceptable representative sampling strategy is difficult to implement
6. From a regulatory point of view, material entering and exiting the reactor is not homogenous. This poses some challenges for the downstream step of the VI, which will have to accommodate this heterogeneity and will make regulatory approval more challenging as the product is of inconsistent concentration and buffer condition during the hold step. A pooling step to homogenize these composition gradients obviates much of the attraction of the plug-flow reactor.
To date there is no commercially available plug-flow solution for low-pH VI, although a series of patents have been filed.4,5
Column-Based Strategies
The stand-out challenge of the plug-flow approach is ensuring all the product stays within the reactor for the entirety of the mandated hold time. This has led to the exploration of other approaches based around column chromatography, including performing the low-pH hold while the product is bound to either a Protein A6 or CEX column7, or alternatively adding a SEC (size exclusion chromatography) step.8
It is difficult to imagine scaling up the SEC column approach, and introducing another chromatography step to achieve the hold time would be adding unacceptable complexity. Performing the low-pH hold using a bind and elute step commits the chromatography media for an hour, adding at least one hour to the cycle time and this has a negative impact on productivity.
For Protein A, it is unclear how column-based inactivation would affect resin lifetime as maintaining mAb binding at the inactivation pH requires the use of high conductivity buffers to retain the mAb hydrophobically.
Continuous In-Process Mixing and Hold
Continuous in-process mixing and hold are a relatively simple automated adaptation of the current manually operated low-pH VI process. As such, it is expected to be the most readily accepted by the regulatory bodies.
It is useful to look at the current batch process to understand why it has not routinely been automated, and on the other side why it needs to be automated. Often, even in a batch process, the Protein A column is cycled two to four times and the eluates captured in a holding vessel. This same vessel is often used the next day for performing the low-pH hold step.
To titrate to the set-point pH, acid is normally pumped in through a J-tube and the system mixed for multiple minutes to ensure homogenization. Then a sample is taken and the pH measured offline using an external pH probe. This is time-consuming, prone to operator error, not entirely reproducible, and potentially damaging to the product; the titration process can take more than an hour, for most of which time the product is close to the set-point pH.
However, transferring this to an automated continuous process and processing in a single biocontainer poses some technical challenges:
1. More than one pooling tank is required as the time for a single elution is likely to be less than the inactivation hold time.
2. To automate the process an immersed pH probe is required, and it must be proven to be accurate.
3. J-tubes are not available for single-use mixers, and addition from the top of the bag is undesirable as it causes splashing and foaming.
4. Conversely, if acid and base are added below the liquid level to minimize splashing and droplet formation, this has the potential to be a source of carryover with back-mixing taking place during the pH hold, thereby changing the pH.
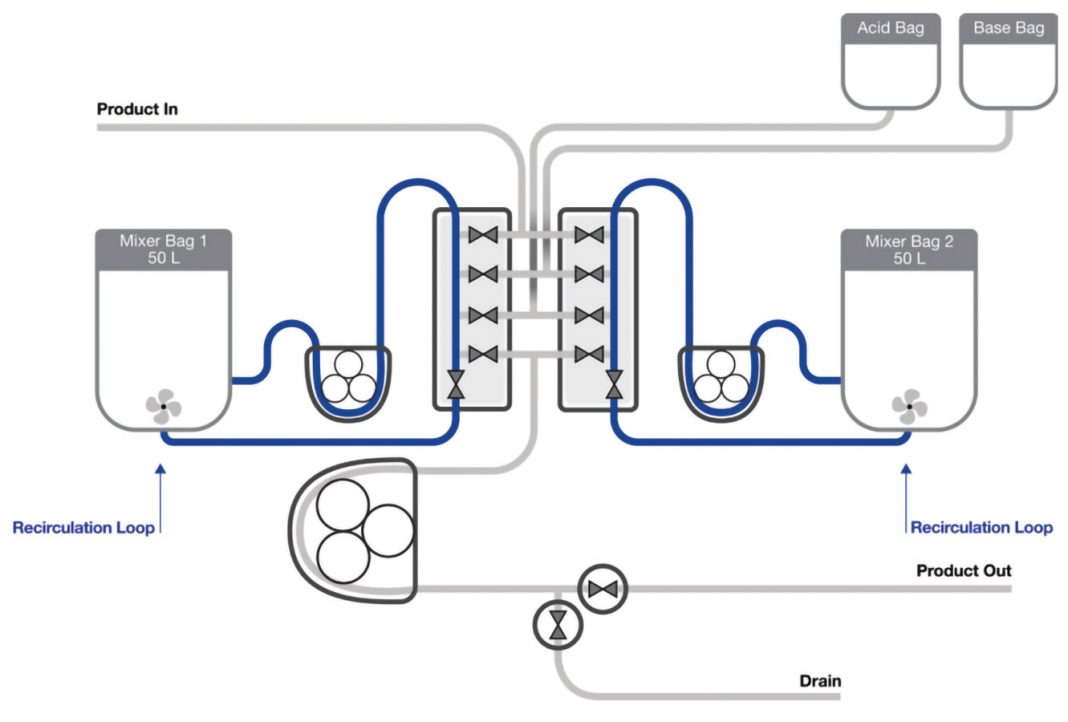
These challenges were tackled via a risk-based approach using failure modes and effects analysis (FMEA), which led the team at Pall Biotech to develop a system that effectively eliminates or mitigates all these risks (Figure 1). To enable the collection and processing of a constant stream of elutions the system has two mixers that are used alternately and asynchronously. The pool of elutions is collected in one of the mixers, in which the complete VI process, acidification, hold, and pH increase take place. While the mixer undertakes the titration and low pH hold, the other mixer is receiving new elutions.
When the inactivation is complete the elution pool is transferred out, and the mixer is ready to collect more elutions, allowing the other mixer to perform the inactivation cycle. This approach facilitates the collection of whole elution peaks, either through a direct communication with the continuous chromatography skid (BioSMB Process) or through weight-change detection.
To screen for pH probes that could be relied upon and that did not exhibit signal drift over time a robotic system was created to mimic the VI process. A probe was identified that is precise, accurate, and exhibits minimal drift (<0.1 pH unit) over 48 hours, and a strategy for calibration developed for longer term running.
To add acid and base below the product liquid level a recirculation loop was implemented that had no hold-ups. A series of tests with Riboflavin, bacteria, and bacteriophage confirmed that the system was hold-up free and no contamination of inactivated product via hanging drops was seen. This is enabled by the combination of the zero hold-up recirculation loop and low point fluid entry.
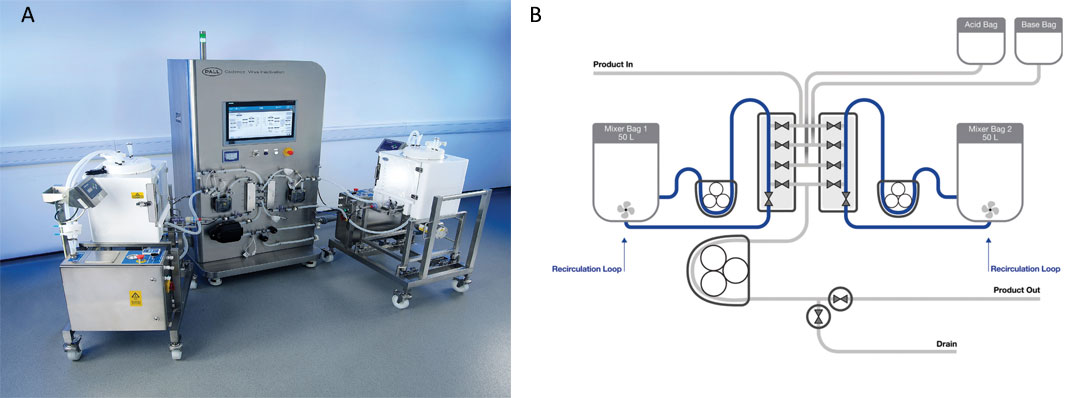
Figure 1. (A) The Cadence VI system, showing the two 50L Allegro mixers and the control module. (B) Schematic of the Cadence VI system.
Conclusion
The Cadence VI system is the first and only commercially available solution for the operation of fully automated semicontinuous low-pH VI. The system adopts the tried and trusted low-pH hold process and has a gamma-irradiated single-use flow-path that contains no hold-ups. This strategy is proven to be robust and reliable, and with the Cadence VI system is now applicable for continuous processing with an alternating tank strategy that is coupled with design elements to minimize carryover and foaming risks with a low operational cost.
Mark Schofield, Ph.D. ([email protected]), is senior research and development manager and David Johnson is senior global product manager at Pall Biotech.
1. Johnson SA, Brown MR, Lute SC, Brorson KA. 2017. Adapting viral safety assurance strategies to continuous processing of biological products. Biotechnol. Bioeng. 114(1): 21–32
2. Zydney AL. 2016. Continuous downstream processing for high value biological products: A review. Biotechnol. Bioeng. 113(3): 465–475.
3. Klutz SL, Lobedann M, Bramsiepe C, Schembecker G. 2016. Continuous viral inactivation at low pH value in antibody manufacturing. Chem. Eng. Process. 102: 88–101.
4. Lobedann M, Klutz S, Klutz S, Kurt SK. 2016. Device and method for continuous virus inactivation. U.S. patent application US20160375159A1.
5. Coffman J, Goby J, Godfrey S, Orozco S, Vogel JH. 2017. Methods, apparatuses, and systems for continuously inactivating a virus during manufacture of a biological product. U.S. patent application US20170037381A1.
6. Bolton GR, Selvitelli KR, Iliescu I, Cecchini DJ. 2015. Inactivation of viruses using novel protein A wash buffers. Biotechnol. Prog. 31: 406–413.
7. Soice N, Hubbard JD, Zhang Y, Hamzik J. 2011. Methods for purifying a target protein from one or more impurities in a sample. U.S. patent application US20110065901A1. C07K 1/22 (20060101); C07K16/00 (20060101); B01D 15/08 (20060101).
8. Arnold L, Lee K, Rucker-Pezzini J, Lee JH. 2018. Implementation of fully integrated continuous antibody processing: Effects on productivity and COGm. Biotechnol. J. doi: 10.1002/biot.201800061. [Epub ahead of print]
Continuous Bioprocessing: Brave Old Challenges with Bold New Technologies
Visions of a Continuous Bioprocess Future
Continuous Processing’s Benefits within Biopharma’s Reach
Vero Perfusion, Packed-Bed Vessels Intensify Vaccine Production
Single-Use Pressure Sensors for Continuous Processing and Leak Testing
A Swiss Army Knife for Modern Biomanufacturing
Next-Generation Processing a Multidisciplinary Pursuit
Next-Generation Bioprocess Techniques