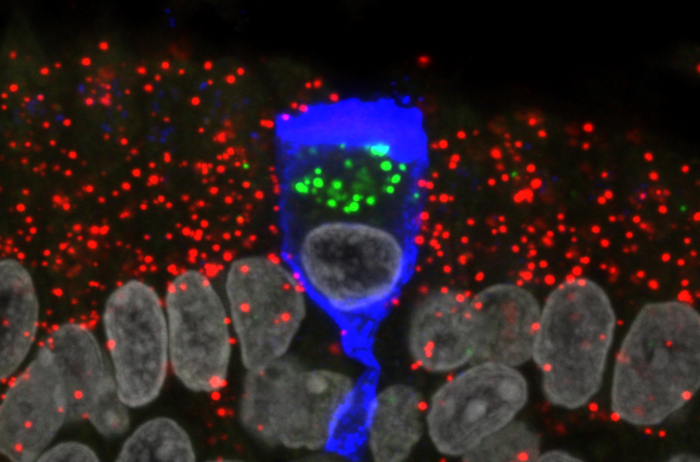
The mechanism underlying anosmia (loss of smell) during a SARS-CoV-2 infection remains unknown. One unresolved question is whether SARS-CoV-2 infects the olfactory nerve, which could provide SARS-CoV-2 with a route of entry to the brain. Now, researchers report that SARS-CoV-2 does not appear to infect the sensory neurons of the olfactory epithelium in COVID-19 patients. Moreover, the team failed to find evidence for infection of olfactory bulb neurons. Instead, the sustentacular (non-neuronal) cells, also known as supporting cells, are the main target cell type for the virus in the olfactory epithelium. Since SARS-CoV-2 spares olfactory sensory neurons and olfactory bulb neurons, it does not appear to be a neurotropic virus.
This work is published in Cell, in the paper, “Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb.”
SARS-CoV-2 uses the ACE2 receptor to bind to cells. ACE2 is expressed by sustentacular cells in the human olfactory epithelium but not by olfactory sensory neurons—the nerve cells that are stimulated by odorants in the inhaled air and that transmit electrical signals to the olfactory bulb. There is no literature about the functions of sustentacular cells in the olfactory epithelium of humans. Studies in laboratory animals suggest that sustentacular cells provide olfactory sensory neurons with a variety of supportive functions, including structural and metabolic support. Both cell types are continuously regenerated from stem cells within the olfactory epithelium throughout the life of an individual.
Tissues of the olfactory mucosa were harvested from deceased COVID-19 patients. As a control, tissue samples were taken from patients who had died from other causes and who were not infected with SARS-CoV-2 at the time of death. The cohort of 85 cases included COVID-19 patients who died a few days after infection with SARS-CoV-2, enabling the team to catch the virus while it was still replicating. Using an endoscope, the physicians collected samples from the respiratory and olfactory mucosa and both olfactory bulbs within 60 to 90 minutes after the death of the patient. “Thanks to this short postmortem interval, the tissue samples were in pristine condition for molecular biology studies,” said Laura Van Gerven, MD, an otorhinolaryngologist at UZ Leuven.
The team of scientists in Frankfurt, led by Mona Khan, PhD, a post-doc at the Max Planck Research Unit for Neurogenetics, used RNAscope to visualize various types of RNA molecules of SARS-CoV-2 within single cells. “Our results show that SARS-CoV-2 infects sustentacular cells in the olfactory epithelium of COVID-19 patients and replicates vigorously within these cells,” said Peter Mombaerts, MD, PhD, director of the Max Planck Research Unit for Neurogenetics.
Spatial, whole-transcriptome analysis, using Digital Spatial Profiler from NanoString Technologies, to analyze sections of the olfactory mucosa of a COVID-19 patient, revealed that infection of sustentacular cells does not alter the expression of olfactory receptor genes in nearby olfactory sensory neurons.

Viral RNA could not be detected in olfactory bulb neurons either. Interestingly, in a third of cases, the researchers detected viral RNA in the meninges surrounding the olfactory bulb, the leptomeninges. In these anatomical locations, the viral RNA may not be present in cells that had been infected with the virus but may stem from virus particles that may have entered the leptomeninges by hitchhiking on the olfactory nerve or via the blood stream. Alternatively, the viral RNA in the leptomeninges may simply represent viral RNA molecules that were floating around in the blood and not packaged in viral particles.
Thus, the results do not support previous suggestions that SARS-CoV-2 can infect nerve cells in humans. In other words, SARS-CoV-2 does not appear to be a neurotropic virus.
The multidisciplinary team postulates that transient olfactory dysfunction in COVID-19 is triggered ty transient insufficient support from sustentacular cells to olfactory sensory neurons. The virus would thus affect olfactory sensory neurons indirectly but without infecting them directly. The pathological consequences of infection of sustentacular cells could vary from patient to patient. The researchers speculate that the immune system may be unable to provide sustentacular cells with full protection from infection, due to their location at the surface of the nasal mucosa. They further speculate that some vaccinated or recovered patients may still lose their sense of smell after exposure to SARS-CoV-2.