In a parallel development to the burgeoning field of optogenetics, scientists at the Salk Institute for Biological Studies have identified a human channel protein (hsTRPA1) and engineered it in mammalian cells in culture and in living animal models to confer cell-specific sensitivity to safe and noninvasive ultrasound stimulation.
Evidence, such as the ability of ultrasound to elicit sensations in the brain through an intact skull, has led to great interest in adapting ultrasound for research and therapy. Prior studies have used ultrasound to direct neuronal functions in humans and other animals, but such efforts have been limited by their lack of specificity.
This is the first study to reveal a molecular mechanism that can be targeted using a safe frequency of ultrasound to regulate neurons in the intact mammalian brain, bringing us a step closer to noninvasive deep brain stimulation, pacemakers, and insulin pumps.
The findings were published in Nature Communications, in an article titled, “Sonogenetic control of mammalian cells using exogenous Transient Receptor Potential A1 channels.”

An earlier study from Chalasani’s team had identified a roundworm protein called TRP-4 that when introduced in the worm’s neurons that normally do not express it, made them sensitive to low-frequency ultrasound. However, TRP4 could not confer ultrasound sensitivity to mammalian cells.
Although work by other groups has identified several ultrasound-sensitive proteins, restricted spatial resolution and impracticality of the conditions used to stimulate these proteins, made them unsuitable for clinical use, particularly for wearable devices.
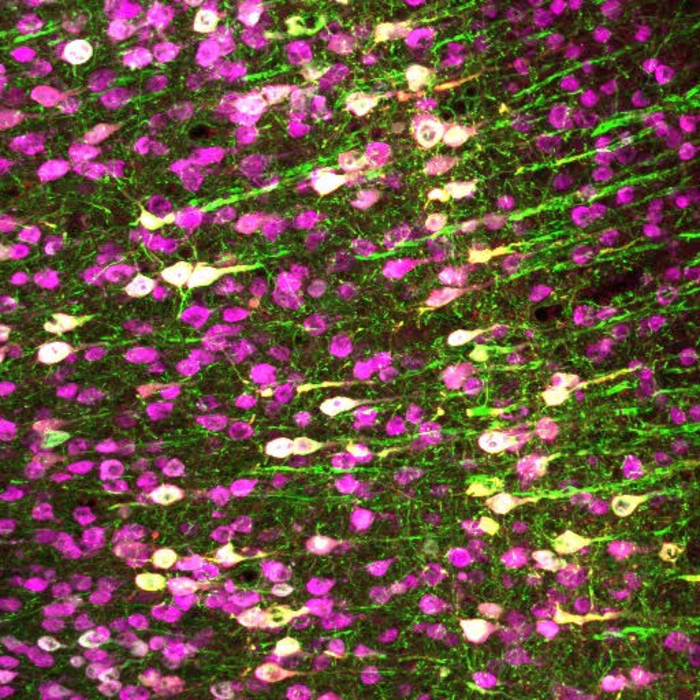
Chalasani decided to use ultrasound at a relatively low frequency (7 MHz) which can be focused to a small volume (107 cubic micrometers) and conducted a comprehensive screen of nearly 300 proteins in HEK cells to identify a mammalian protein that responded to this low ultrasound frequency.
Eventually, the team identified the channel protein TRPA1 that when introduced in HEK cells made them sensitive to ultrasound at a frequency of 7 MHz. Normally, TRPA1 is found in a variety of cells including neurons and responds to the presence of noxious compounds. The team used gene therapy to introduce the human TRPA1 gene into a group of neurons in live mouse brains and demonstrated selective neural sensitivity to ultrasound.
Marc Duque, a Salk exchange student and co-first author on the paper, who along with this team was surprised by the finding, said, “TRPA1 has been well-studied in the literature but hasn’t been described as a classical mechanosensitive protein that you’d expect to respond to ultrasound.”
At present, deep brain stimulation used to treat Parkinson’s and epilepsy involves surgically implanting electrodes in the brain, to activate subsets of neurons. Chalasani believes sonogenetics in conjunction with gene therapy could offer a noninvasive and safer alternative.
“Gene delivery techniques already exist for getting a new gene—such as TRPA1—into the human heart,” Chalasani said. “If we can then use an external ultrasound device to activate those cells, that could really revolutionize pacemakers.”
Chalasani’s team is currently investigating the exact mechanism of TRPA1’s ability to detect ultrasound. Corinne Lee-Kubli, PhD, co-first author of the paper and a former postdoctoral fellow at Salk said, “We hope to determine exactly what parts of TRPA1 contribute to its ultrasound sensitivity and tweak them to enhance this sensitivity.”
Uncovering these basic biological insights will enhance the value of the finding for research and therapeutic applications. In addition, the team is conducting screens to detect proteins that block the ability of cells to respond to ultrasound, so that the technology can be controlled bidirectionally.