Researchers at UT Southwestern (UTSW) and Indiana University have used genetic engineering techniques to trigger the production of functional nerve cells from scar-forming cells in mouse spinal cords, in response to spinal cord injury (SCI). The studies showed that neurogenic reprogramming of the spinal NG2 glial cells led to functional improvements in the animals after SCI, with the newly formed neurons rebuilding neuronal circuits.
The team hopes the achievement could point to new therapeutic approaches that could one day help the hundreds of thousands of people worldwide who suffer a spinal cord injury each year. The researchers, led by Chun-Li Zhang, PhD, professor of molecular biology and a W.W. Caruth, Jr. scholar in biomedical research at UTSW, reported their results in a paper in Cell Stem Cell, in which they concluded: “Our results reveal a cellular and molecular mechanism underlying neural injury-induced cell plasticity that can be exploited for adult neurogenesis and relay formation in a region that has largely lost the ability to regenerate.” Their report is titled, “In vivo reprogramming of NG2 glia enables adult neurogenesis and functional recovery following spinal cord injury.”
Cells in some body tissues proliferate after injury, replacing dead or damaged cells as part of healing. The brain itself does have limited capacity to produce new nerve cells, and this is possible thanks to progenitor cells that turn on distinct regenerative pathways. Adult neurogenesis in the brain plays critical roles in maintaining homeostasis and responding to neurological conditions, including injuries, Zhang and colleagues explained.

Using the knowledge of limited brain neurogenesis as inspiration, the researchers looked for cells that might have similar potential for regeneration in the spinal cord. Working with a mouse model of spinal cord injury, they looked in the animals’ injured spinal cords for a marker, DCX, that is normally found in immature neurons. “DCX is normally expressed in neuroblasts and immature neurons and can serve as a reliable marker for adult neurogenesis,” the team wrote. “It is highly expressed in the developing spinal cord but completely turned off in the adult.” Interestingly, they identified this marker in the spinal cord specifically after injury, and also tracked the marker to the non-neuronal NG2 glia cells, which produced it in response to SCI.
NG2 glia serve as progenitors for oligodendrocytes, cells that produce the insulating myelin layer that surrounds neurons. NG2 glia are also recognized as forming glial scars following injury. “In response to injury, NG2 glia increase their numbers and become a major component of the glial scar,” the scientists noted.
But the work by Zhang’s team also showed that when the spinal cord in mice was injured, these glial cells transiently adopted molecular and morphological markers of immature neurons. To determine what caused the NG2 glia to change, the researchers focused on SOX2, a stem cell protein induced by injury. “To understand how SCI induces cell reprogramming, we focused on SOX2, a stem cell factor essential for neurogenesis and neural development,” the authors explained. “… our immunohistochemistry showed a 3.6-fold increase in the number of SOX2+ cells and the intensity of SOX2 expression in each cell surrounding the lesion site of the adult mouse spinal cord … Most intriguingly, nearly all (94%) of the SCI-induced DCX+ cells co-expressed SOX2.”
When the scientists then genetically manipulated NG2 cells to inactivate the gene that makes the SOX2 protein, they saw far fewer immature neurons in the days following SCI, suggesting that SOX2 plays a key role in helping NG2 glia make these new immature neurons. However, they also found that even with normal levels of SOX2, these immature neurons never matured into replacements for those affected by the injury. “SCI only induces a transient phenotypic switch of NG2 glia to DCX+ cells, which eventually fail to become mature neurons,” they wrote.
Zhang and his colleagues then used a different genetic manipulation technique to make NG2 glia overproduce SOX2. They found that in the weeks after spinal cord injury, mice with SOX2-overproducing NG2 glia generated tens of thousands of new mature neurons. Further investigation showed that these neurons integrated into the injured area, making the new connections with existing neurons that are necessary to relay signals between the brain and body. Even more promising, suggested Zhang, is that this genetic engineering led to functional improvements after spinal cord injury.
Encouragingly, animals engineered to overproduce SOX2 in their NG2 glia performed markedly better on motor skills weeks after spinal cord injury, when compared with animals that produced normal amounts of SOX2. The reasons for this improved performance seemed to be multifold. Not only had the animals generated neurons that appeared to take over for those damaged during injury, but they also had far less scar tissue at the injury site that could hinder recovery, Zhang explained. “…reprogramming of NG2 glia leads to generation of new neurons and reduction of glial scars, both of which may contribute to functional improvements after SCI,” the team noted.
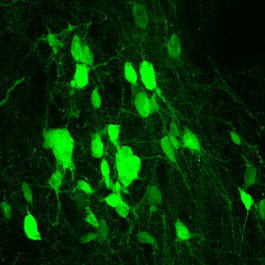
“The field of spinal cord injury has extensively researched trying to heal the damage with stem cells that produce new neurons, but what we’re proposing here is that we may not need to transplant cells from the outside,” Zhang said. “By encouraging NG2 glia to make more SOX2, the body can make its own new neurons, rebuilding from within.”



