If you want to know if a medical field is progressing, you can start by checking a couple of obvious indicators: the number of clinical trials, and the growth projections offered by market researchers. Both of these indicators suggest that regenerative medicine is moving smartly. Last January, at the 2023 Cell and Gene State of the Industry Briefing, the Alliance for Regenerative Medicine’s CEO, Timothy D. Hunt, said that more than 2,200 active clinical trials in regenerative medicine were in progress. And last February, Grand View Research announced that it had projected that the regenerative medicine market would sustain a growth rate of 15.7%, and that the market would be worth $180 billion by 2030.
There is yet another indicator: The speed with which a field widens its scope. Even though regenerative medicine is fairly new—the field is generally considered to have started with the stem cell advances of the 1990s and 2000s—it now encompasses a broad range of activities. According to the Alliance for Regenerative Medicine, these activities include “gene therapies, cell therapies, and tissue-engineered products intended to augment, repair, replace, or regenerate organs, tissues, cells, genes, and metabolic processes in the body.”
Regenerative medicine is not only expanding its scope, but it is also becoming increasingly commercial. These developments are being driven, in part, by innovative companies, such as the ones mentioned in this article. These companies include Avery Therapeutics, which develops skin cell–derived treatments for chronic heart failure; Regenative Labs, which provides Wharton’s jelly allografts that can counter the degeneration of load-bearing joints and intervertebral discs; CollPlant Biotechnologies, which manufactures virgin human collagen scaffolds at scale; and Aivita Biomedical, which applies stem cell purification technology (for personalized vaccines against cancer and COVID-19) and retinal organoid technology (for treatments to restore vision).
Getting to the heart of the matter
Avery Therapeutics has taken a different path to fix broken hearts than other players in the field. Rather than trying to construct new heart tissue that will directly replace tissue damaged in an infarct, the company is focused on microtransplanting engineered cardiomyocytes onto damaged hearts to catalyze an immune response that could help reduce scarring and promote functional healing.
This approach reflects the company’s current emphasis on fixing, rather than replacing, damaged tissues and organs. “I really don’t like the word regeneration,” says Jordan Lancaster, PhD, Avery’s CEO. “It’s used in about 50 different contexts, which can be very misleading. So, I just like to use the word ‘repair.’”
Avery is focused on breaking the downward spiral of chronic heart failure. Successfully treating a heart attack still leaves a patient with some damaged, scarred, nonfunctional heart tissue, the result of pro-fibrotic macrophage activity in the aftermath of the infarct. The rest of the heart enlarges and changes to compensate for that lack of function, a process that increases the likelihood of future heart failure, according to Lancaster.
“What’s crazy is our management strategies today work to reduce the burden, the amount of work that the heart has to do,” he says. “An example of that would be diuretics—you’re treating patients’ hearts by making them pee more.” And over the long term, the treatment for heart failure is a heart transplant, which is costly, poses risks for many patients, and requires ongoing immunosuppression.
Instead, Avery has developed what it calls MyCardia, a microtransplant consisting of human induced pluripotent stem cell–derived cardiomyocytes and human neonatal fibroblasts in a bioengineered scaffold forming a patch about 5 cm in diameter. Delivered via catheter and laid down on the heart, the MyCardia patch appears to recruit different macrophage populations seen in noncardiac tissue. The patch can take the healing process further and restore heart function rather than leaving scars behind.
An immune response is essential to the process, and so Avery has conducted its preclinical work accordingly. The company has implanted MyCardia only in immune-intact animal models (mouse, rat, and swine). Lancaster believes that this could be why the company’s researchers haven’t seen any arrhythmias of the kind seen in similar studies where animals were immunosuppressed. “We actually tried to cause arrhythmia,” he remarks, “but we found that the hearts were more stable.”
Lancaster says that he hopes to move MyCardia into clinical trials within about 24 months. In the meantime, the company is focused on work that he believes is just as crucial to the field as clinical trials—work to overcome the challenges to commercialization. That MyCardia can be implanted via catheter in 20 minutes, versus the hours necessary for other procedures, shaves costs off of implementation.
Avery is also focused on supply chains and delivery. Lancaster says that MyCardia, which is stable for five days at room temperature, can also be cryopreserved and then thawed without loss of function. “Shipping stability and time to transplant—these are really important questions that aren’t asked a lot,” Lancaster points out. “Medicine should be making things cheaper, faster, and more accessible.”
Protecting joints
Regenative Labs, based in Pensacola, FL, already has regenerative medicine products in the marketplace, largely human cells, tissues, or cellular or tissue-based products (HCT/Ps). And unlike Avery’s products, these are not engineered tissues. Instead, they are minimally processed derivatives of Wharton’s jelly, a gelatinous complex of connective tissue material derived from donated umbilical cords.
“We don’t manipulate or clone any tissue,” says Tyler Barrett, Regenative’s CEO. “We take tissue from healthy, live, planned C-section babies, sourced from consenting mothers, with all the necessary precautions put into place regarding tissue health.”
Rather than triggering an immune response or attempting to regrow tissue, the Regenative approach simply seeks to replace the cushioning properties of joint connective tissue lost in joint dysfunction.
Regenative recently announced preliminary data from a retrospective study of more than 50 patients with sacroiliac joint dysfunction treated with Regenative’s injectable Wharton’s jelly products. Patients showed a more than 35% improvement in pain and stiffness scores following the treatment. Barrett believes this shows that there’s an alternative to the standard of care for sacroiliac joint disease—care that he says is largely focused on making masking symptoms.
“The current standard for care of sacroiliac joint addresses the symptoms involved, with pain medication, braces, or sacroiliac joint fusion,” Barrett asserts. “That’s about it in terms of standard care. There’s really not much.”
Regenative’s approach is just one slice of the regenerative medicine field, but it is illustrative of the new way of thinking prevalent across different companies and researchers. “This represents a significant shift in how we approach medical pathology,” Barrett stresses. “Rather than assigning a novel term to describe the deterioration of the tissue, we simply acknowledge the absence of tissue and seek to replace it. Currently, the key challenge is focused around raising public awareness that this option exists.”
Using plants to grow connective tissue
At Israel-based CollPlant Biotechnologies, “connective tissue” has two senses. One is literal. The company makes connective tissue. The other is figurative. The company aspires to be the connective tissue between regenerative medicine applications. Founded in 2005, CollPlant’s core technology is the ability to grow recombinant human type one collagen in tobacco plants genetically engineered to express the human genes for the collagen protein.
“Collagen is the ideal scaffolding molecule for regenerative medicine applications,” says Yehiel Tal, CollPlant’s CEO. “You can use it for any type of tissue or organs that you want to regenerate.”
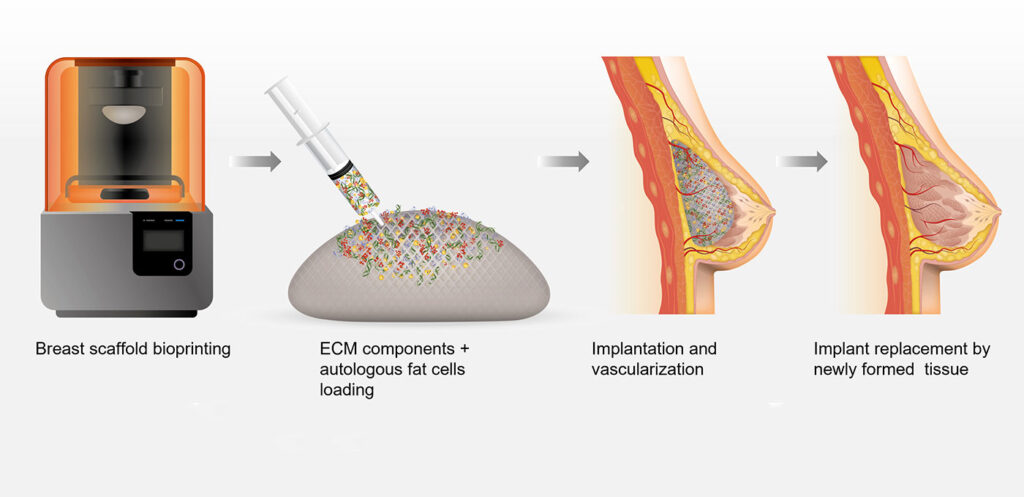
CollPlant provides a recombinant human Type I collagen product called rhCollagen. According to Tal, rhCollagen won’t elicit an immune response like collagen from pigs, cattle, or human cadavers. That’s important for the products CollPlant is developing. For applications in 3D bioprinting, CollPlant is developing bioinks. For applications in medical aesthetics, the company is developing breast implants and dermal fillers.
The implants are meant to do more than fill space. They are designed to recruit cells and dissolve over about 6 to 12 months, depending on the size of the implant. Ultimately, they are meant to be replaced with naturally grown breast tissue.
CollPlant recently completed a successful preclinical study of rhCollagen-based breast implants in pigs. And now the company plans to undertake another animal study with its implants, this time using full-sized structures in pigs. The company hopes that its implants will eventually support applications such as human breast reconstruction or augmentation.
Although breast implants are an application specific to medical aesthetics, Tal says that CollPlant hopes to position itself as the supplier of connective tissue for all kinds of regenerative medicine applications both in and out of the house. He adds that CollPlant’s implants and bioinks demonstrate the utility of rhCollagen for everything from drug testing to whole organ bioprinting. Pursuing applications such as these may help CollPlant become, in Tal’s words, “a significant player from the collagen point of view as an ideal substrate for these applications.”
Applying lessons from early stem cell treatments
Before founding Aivita Biomedical in 2016, Hans Keirstead, PhD, was already established in regenerative medicine. And he had already learned that promising developments in the laboratory can take years to come to fruition in the marketplace. In 2003, as a new professor of anatomy and neurobiology at the University of California, Irvine, Keirstead developed a technique to derive 99.6% pure oligodendrocytes from embryonic stem cells (J. Neurosci. 2005; 25(19): 4694–4705).
Oligodendrocytes myelinate nerve fibers, providing electrical insulation to help them conduct nerve impulses. Using cultured oligodendrocytes, Keirstead tested his theory that remyelination of the nerves surrounding a spinal cord injury might restore some degree of patient function. He ended up making paralyzed rats walk again. “That made for one hell of a good video,” he recalls.
These promising preclinical results encouraged Geron to launch a 26-patient study in human quadriplegics with spinal cord injuries. In this study, 100% of patients who received injections around their injury site saw restored sensation and motor functions in their upper limbs. “The program,” Keirstead recalls, “was going tremendously well.”
But then the study was suspended by Geron, which complained of “the current environment of capital scarcity and uncertain economic conditions.” According to Keirstead, the company had decided that the treatment would never earn back the investment necessary to bring it to market.
“Remember, a blockbuster has 15% market penetration,” Keirstead explains. “If we took 100% of the market, that would amount to just $300 million annually.” And according to Keirstead, it would have taken a billion dollars to bring the treatment to market. Any endeavor with numbers such as these, he observes, is “dead before it starts.”
It was a tough lesson, but an important one: The science can be amazing, but it won’t get into patients if it’s not economically viable. Keirstead says that he recognized the need to “work on a large market indication and hook spinal cord injury on the back of that.”
This is the work that Keirstead is doing today at Aivita, which he serves as CEO. Under his leadership, the company is developing a personalized, stem cell–based cancer treatment, as well as retinal regeneration technology. According to Keirstead, the company’s programs address markets that could be as large as $6 billion to $20 billion.
Still in preclinical development, Aivita’s retinal regeneration program is creating retinal organoids created from human embryonic stem cell lines. When implanted in rodent models, these organoids successfully restored the animals’ visual acuity (Front. Neurosci. 2021; 15: 752958). The company also completed a Phase II study of an immunotherapy for glioblastoma showing evidence of improved progression-free survival (J. Oncol. Res. Ther. 2022; 7: 10149.).
“There have only been three [glioblastoma] treatments that have been approved, and each of them increased survival by only 10 to 15%,” Keirstead points out. “We did it by 50%.”
The treatment, called AV-GBM-1, is produced by culturing and purifying cancer stem cells taken from a patient’s tumor and then training the patient’s own dendritic cells on the cancer stem cells. “[This] immunotherapy mounts an immune response only against your tumor-initiating cells or your cancer stem cells,” Keirstead says. The company is currently raising funding for a Phase III trial.
In all of Aivita’s work, Keirstead attempts to keep in mind the early lesson of his spinal cord injury research, which is that scientifically viable or even clinically viable is not the same thing as commercially viable. This lesson, which is important for the regenerative medicine field in general, certainly applies to the production of three-dimensional retinal implants. With respect to such implants, Keirstead notes that other groups have found that the “cost of goods hovers around $300,000,” and that the “implants take a long time to grow.”
Aivita is focused on growing retinal implants at a fraction of that cost and on ensuring that growth occurs much faster. “They’re not only clinically viable, they’re also commercially viable,” Keirstead declares. “That’s something that the academic sphere doesn’t really appreciate.”