Although research studies have demonstrated that taking probiotics can reduce gastrointestinal side effects from antibiotics, there has been debate over whether taking probiotics alongside antibiotics can also preserve the diversity and composition of microbes in the gut. Some healthcare professionals are reluctant to recommend probiotics alongside antibiotics for fear of further altering the delicate balance of microbes in the patient’s gut.
Scientists, who have just published a new paper, “Effect of adding probiotics to an antibiotic intervention on the human gut microbial diversity and composition: A systematic review” in the Journal of Medical Microbiology, say their work provides the first systematic review to assess the effect of taking probiotics alongside antibiotics on the diversity and composition of the human gut microbiome. Authored by researchers from the School of Medical and Health Sciences at Tecnológico de Monterrey, the University of Texas, and Texas Christian University, the review evaluates trends across 29 studies published over the past seven years.
The authors, whose goal was to evaluate whether the addition of probiotics is capable of reverting the changes in alpha diversity and gut microbial composition commonly observed in adult participants receiving antibiotics, found that taking probiotics alongside antibiotics can prevent or lessen some antibiotic-induced changes to gut microbiome composition. Probiotics can also help protect species diversity and even restore the populations of some friendly bacteria such as Faecalibacterium prausnitzii, which reduces inflammation and promotes a healthy intestinal barrier.
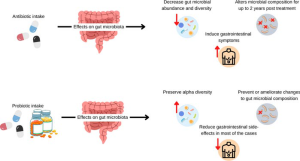
“A total of 29 articles (n=11 antibiotics, n=11 probiotics, and n=7 antibiotics+probiotics) met the inclusion criteria. The lack of standardization of protocols to analyze the gut microbial composition and the wide range of selected antibiotics/probiotics complicated data interpretation; however, despite these discrepancies, probiotic co-administration with antibiotics seemed to prevent some, but not all, of the gut microbial diversity and composition changes induced by antibiotics, including restoration of health-related bacteria such as F. prausnitzii.
“Addition of probiotics to antibiotic interventions seems to preserve alpha diversity and ameliorate the changes to gut microbial composition caused by antibiotic interventions.”
“Even though we haven’t come up with a single definition of what is a healthy gut microbiome, one of the constant things we observe in healthy people is that they have a higher level of diversity and more variety of bacteria in the gut,” explained Elisa Marroquin, PhD, assistant professor at Texas Christian University, and co-author of the paper. “When participants take antibiotics, we see several consistent changes in some bacterial species. But when treatment was combined with probiotics, the majority of those changes were less pronounced, and some changes were completely prevented. Considering the human data available up to this point, there does not seem to be a reason to withhold a prescription of probiotics when antibiotics are prescribed.”


