People with panic disorder may frequently and unexpectedly experience symptoms that include overwhelming fear, sweaty palms, shortness of breath, and rapid heart rate. Creating a map of the regions, neurons, and connections in the brain that mediate these panic attacks could provide guidance for developing more effective panic disorder therapeutics.

“We’ve been exploring different areas of the brain to understand where panic attacks start,” said Sung Han, PhD, associate professor at Salk. “Previously, we thought the amygdala, known as the brain’s fear center, was mainly responsible—but even people who have damage to their amygdala can still experience panic attacks, so we knew we needed to look elsewhere. Now, we’ve found a specific brain circuit outside of the amygdala that is linked to panic attacks and could inspire new panic disorder treatments that differ from current available panic disorder medications that typically target the brain’s serotonin system.”
Han is the senior author of the team’s published paper in Nature Neuroscience, titled, “A pontomesencephalic PACAPergic pathway underlying panic-like behavioral and somatic symptoms in mice,” in which the team concluded that their findings “… delineate a new neural mechanism underlying panic-specific behavioral and somatic symptoms, which may facilitate the identification of therapeutic targets for the treatment of panic disorder.”
Anxiety disorders constitute the most common class of psychiatric diseases, and encompass post-traumatic stress disorder, generalized anxiety disorder, phobic disorders and panic disorder, the authors explained.

However, the team continued, “Neural mechanisms underlying these unique symptoms are not completely understood.” To begin sketching out a panic disorder brain map, the researchers looked at a part of the brain called the lateral parabrachial nucleus (PBL) in the pons (part of the brain stem), which is known as the brain’s alarm center. Interestingly, this small brainstem area also controls breathing, heart rate, and body temperature.
“The PBL regulates autonomic functions (for example, cardiorespiratory activity, body temperature), relays multimodal aversive sensory signals to the amygdala and, compellingly, coordinates breathing rate with anxiety,” the authors pointed out. Furthermore, they noted. the PBL is activated by panicogenic conditions in rodents, and possibly in humans. “The PBL is therefore a promising candidate for a neural substrate of panicogenesis.”
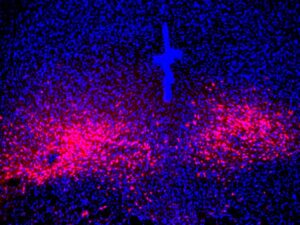
As part of their study the team turned to a mouse model of panic attacks to confirm and expand their proposed map. Their investigations, including cell-type and projection-specific circuit monitoring, manipulation and mapping techniques, implicated the PBL in generating panic and bringing about emotional and physical changes.
“Emotional and stress-related behaviors have been associated with PACAP-expressing neurons in the past,” says co-first author Sukjae Kang, PhD, senior research associate in Han’s lab. “By mimicking panic attacks in the mice, we were able to watch those neurons’ activity and discover a unique connection between the PACAP brain circuit and panic disorder.”
They found that during a panic attack, PACAP-expressing neurons became activated. Once activated, they release PACAP neuropeptide messenger to another part of the brain called the dorsal raphe (DR), where neurons expressing PACAP receptors reside. The released PACAP messengers activate those receptor neurons, thereby producing panic-associated behavioral and physical symptoms in the mice. This connection between panic disorder and the PACAP brain circuit is an important step forward for mapping panic disorder in the brain, Han says.
The team also found that by inhibiting PACAP signaling, they could disrupt the flow of PACAP neuropeptides and reduce panic symptoms—a promising finding for the future development of panic disorder-specific therapeutics. “Chemogenetic or pharmacological inhibition of downstream PACAP receptor-expressing dorsal raphe neurons abolished the panic-like symptoms,” they wrote. Taken together, these findings demonstrate that the PACAPPBL→DR signaling pathway mediates panic disorder-specific behavioral and somatic symptoms … Therefore, the PACAPPBL → PAC1RDR signaling pathway is an ideal target for new therapeutic interventions for panic disorder.”
The team will look to explore PACAP-expressing neurons and PACAP neuropeptides as novel druggable targets for panic disorder. Additionally, they are hoping to further build out their map of panic disorder in the brain to see where the PACAP receptor-producing neurons in the dorsal raphe send their signals, and how other anxiety-related brain areas interact with the PACAP panic system. “
We found that the activity of PACAP-producing neurons in the brain’s parabrachial nucleus is inhibited during anxiety conditions and traumatic memory events—the mouse’s amygdala actually directly inhibits those neurons,” noted Han, who is also the Pioneer Fund Developmental Chair at Salk. “Because anxiety seems to be operating conversely to the panic brain circuit, it would be interesting to look at the interaction between anxiety and panic, since we need to explain now how people with anxiety disorder have a higher tendency to experience panic attack.”



