Nerve tissue engineering is an application of tissue engineering in peripheral nerve regeneration, and tissue-engineered nerve grafts, like other tissue-engineered constructs, are typically composed of a nerve scaffold combined with cellular and/or molecular components. A team of researchers from RMIT University in Australia, working with scientists from India and Bangladesh have developed a neuron-growing ink that uses the body’s own electrical signals to precisely guide the growth of nerve cells. Their work opens a new avenue in nerve engineering.
Their findings, “Three-dimensional directional nerve guide conduits fabricated by dopamine-functionalized conductive carbon nanofiber-based nanocomposite ink printing,” were published in RSC Advances.
“A potential issue in current nerve guides is that they do not transmit electrical nerve impulses between the distal and proximal end of an injured nerve, i.e., a synapse,” noted the researchers. “Conductivity is a desirable property of an ideal nerve guide that is being considered for peripheral nerve regeneration. Most conductive polymers reported for the fabrication of tissue engineering scaffolds, such as polypyrrole and polyaniline, are non-biodegradable and possess weak mechanical properties, and thus cannot be fabricated into 3D structures. Herein, we have designed a new nanocomposite material composed of dopamine, carbon nanofibers (CNF), and polycaprolactone (PCL) for the fabrication of nerve conduits, which facilitates the growth and migration of neurons toward the targeted end of an injured nerve.”
“Nerve cells need to be meticulously guided to regrow between the broken ends of a nerve—if they just build up anywhere they will cause more pain or sensory problems,” RMIT University’s Shadi Houshyar, PhD, explained. “With our bioconductive ink, we can concentrate the neuron growth where we need it. Our research is in early stages but with further development, we hope one day to enable damaged nerves to be fully reconnected, to improve the lives of millions of people worldwide.”
Unlike other tissues in the body, peripheral nerve regeneration is slow and usually incomplete. Less than half of patients who undergo nerve repair after injury regain good to excellent motor or sensory function The new nerve-regenerating ink combines dopamine with a conductive carbon nanofiber and polymer.
“Using conductive materials allows free movement of electrons, stimulates cell growth, and helps connect injured neural tissue,” said Houshyar, a vice-chancellor’s research fellow in the RMIT School of Engineering.
The researchers also developed a biocompatible scaffold, so the ink could be printed in lines and tested with human cells. The researchers observed the printed lines supported neural cell attachment and migration. Cell differentiation was also boosted, with the neural cells becoming more specialized as they grew along the lines.
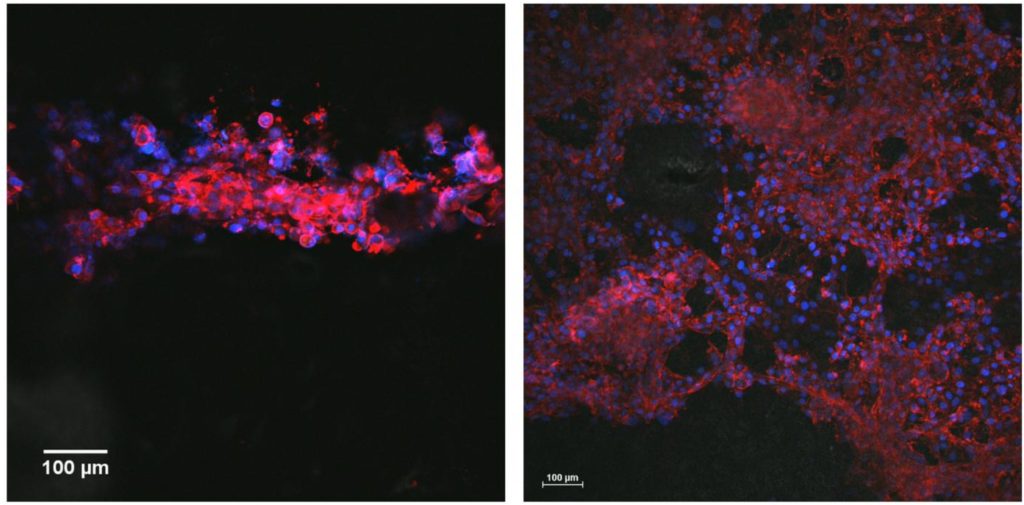
The researchers are looking forward to the future and testing their ink and scaffold in preclinical animal trials.
“Our end goal is a nerve engineering solution that can direct the growth of the right nerve cells in the right places,” she said.
“We’re also keen to investigate how we can expand the potential uses of this technology, for speeding up wound healing and improving patient recovery.”



