Surgeons and neuroscientists in the U.S. have successfully grafted human neural progenitor cells (NPCs) into rhesus monkeys with spinal cord injuries (SCIs). The grafts sent out hundreds of thousands of new human axons across the damaged tissue to connect with functional neuronal circuits in the animals’ spinal cords, resulting in improved limb function. The work, headed by a team at the University of California, San Diego (UCSD), represents a significant bridge between earlier work in rodents, and the potential to use similar procedures in humans.
The researchers, led by Mark Tuszynski, M.D., Ph.D., professor of neuroscience and director of the University of California (UC), San Diego Translational Neuroscience Institute, claim their achievement represents a major step toward addressing barriers that prevent the translation of prior work in rodents into methods that could feasibly be used in humans. “We seem to have overcome some major barriers, including the inhibitory nature of adult myelin against axon growth,” Tuszynski notes. “Our work has taught us that stem cells will take a long time to mature after transplantation to an injury site, and that patience will be required when moving to humans. Still, the growth we observe from these cells is remarkable—and unlike anything I thought possible even ten years ago. There is clearly significant potential here that we hope will benefit humans with spinal cord injury.”
The primate studies are reported in Nature Medicine, in a paper entitled “Restorative Effects of Human Neural Stem Cell Grafts on the Primate Spinal Cord.”
Despite 30 years of research, the ability to achieve abundant, long-distance regeneration of injured axons has remained “elusive,” the researchers report. This inability to promote axonal regeneration after SCI is probably related to multiple contributing factors, including the inhibitory nature of the extracellular matrix that develops around the injury site, the inhibitory nature of myelin proteins, and a lack of growth-promoting factors such as neurotrophins.
To date, much work has been carried out in rodents to address these obstacles.“For more than three decades, spinal cord injury research has slowly moved toward the elusive goal of abundant, long-distance regeneration of injured axons, which is fundamental to any real restoration of physical function,” comments Tuszynski. “While there was real progress in research using small animal models, there were also enormous uncertainties that we felt could only be addressed by progressing to models more like humans before we conduct trials with people.”
The UCSD-led team had previously developed methods for grafting neural stem cells to sites of SCI in rodents, resuting in electrophsyioogical and functional improvements, even after severe SCI. However, as the researchers point out, what works in rodents may not work in primates. “Indeed, a number of experimental therapies showing efficacy in rodent models may be ineffective or impractical in primates; these therapies may fail for numerous reasons, including differences in intrinsic biology, small effect sizes, poor safety and tolerability, and lack of scalability to humans of methods developed in rodents.”
“We discovered, for example, that the grafting methods used with rodents didn't work in larger, nonhuman primates,” Dr. Tuszynski notes. “There were critical issues of scale, immunosuppression, timing, and other features of methodology that had to be altered or invented. Had we attempted human transplantation without prior large animal testing, there would have been substantial risk of clinical trial failure, not because neural stem cells failed to reach their biological potential but because of things we did not know in terms of grafting and supporting the grafted cells.”
Acknowledging the importance of primate studies to address existing issues prior to human trials, the researchers developed a protocol for successfully grafting NPCs into primates. The primate protocol differed from rodent protocols in a number of aspects. These included modifications to the grafting matrix, physical changes to the grafting technique, and more extensive immunosuppression using three drugs.
Using the modified protocol, the team delivered human NPC grafts to nine adult rhesus monkeys. The animals received the grafts two weeks after SCI injury, which the researchers suggest is “a clinically relevant time period in which humans might be expected to medically stabilize before undergoing neural stem cell therapy.” Each animal received 20 million human NPCs, grafted directly into the injury site. The grafted NPC cells were suspended in a cocktail of growth factors to support their survival.
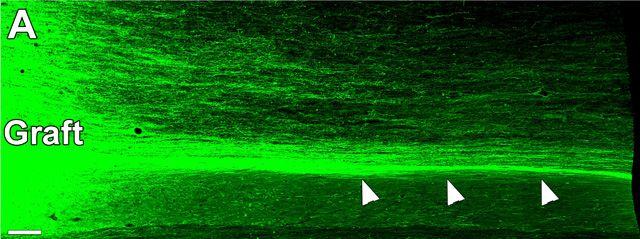
Over the next nine months, the grafts integrated with the host spinal cord, and even within the first two months neuronal markers were expressed. Most notably, human axons extended out from the grafts in what the researchers describe as “extraordinary numbers and over long distances.” Up to 150,000 graft-derived human axons were present, with some reaching distances of up to 50 mm from the graft site. Importantly, the axons extended through the host’s white matter, and synapsed with distal grey matter, suggesting that adult myelin does not inhibit the growth of human axons. “Here we show that axons emerging from human neural stem cells extend abundant axons through white matter, overcoming a major limiting factor in the regeneration field,” the team states.
Along with confirmation that human axons could grow out of the grafts across the site of damage and connect with the host’s existing, undamaged circuitry, the researchers also hoped that the host animal’s axons would extend into the grafts. “In theory, human neural stem cell implantation into a lesion cavity aims to re-establish a neuronal relay across a site of SCI,” they note. “For this to occur, host axons must regenerate into neural stem cell grafts, and neural stem cells must extend axons out of these grafts and into the host spinal cord.”
Encouragingly, immunolabeling studies showed that the host axons had also regenerated into the myelin-free human neural stem cell grafts. “We now report for the first time to our knowledge the regeneration of primate corticospinal axons into human NPC grafts,” the authors claim. “Corticospinal axons readily crossed the host–graft interface to penetrate distances up to 500 micrometers into the graft,”
Most promisingly, monkeys with surviving grafts demonstrated greater improvements in forelimb function, measured through multiple tasks, than surviving animals with nonsurviving grafts. “Function among monkeys with nonsurviving grafts…exhibited stability or only partial spontaneous improvement up to 4–8 weeks postinjury but then reached stability without subsequent overall improvement.…In contrast, among monkeys with surviving grafts, the initial 4- to 8-week period of functional loss or partial spontaneous improvement after lesion was followed by a second period of subsequent improvement 10 weeks after lesion.” The authors note that even greater functional improvements may have been seen if the study had been extended. “It is possible that longer observation periods could result in greater recovery,” they write in their discussion.
“Axon regeneration, synapse formation, myelination—these all take time, and are critical for neural function,” explains Ephron S. Rosenzweig, Ph.D., first author of the Nature Medicine paper, who is an assistant adjunct professor in Tuszynski’s lab. “Grafts, and the new circuitry they were part of, were still maturing at the end of our observations, so it seems possible that recovery might have continued.”
Dr. Tuszynski notes that more work will be needed before clinical trials can be envisaged. This will include the production of an approved candidate human neural stem cell line and further carrying out further safety studies. The group is also continuing to investigate ways of enhancing the the growth, distance, and functionality of the regenerated cells.