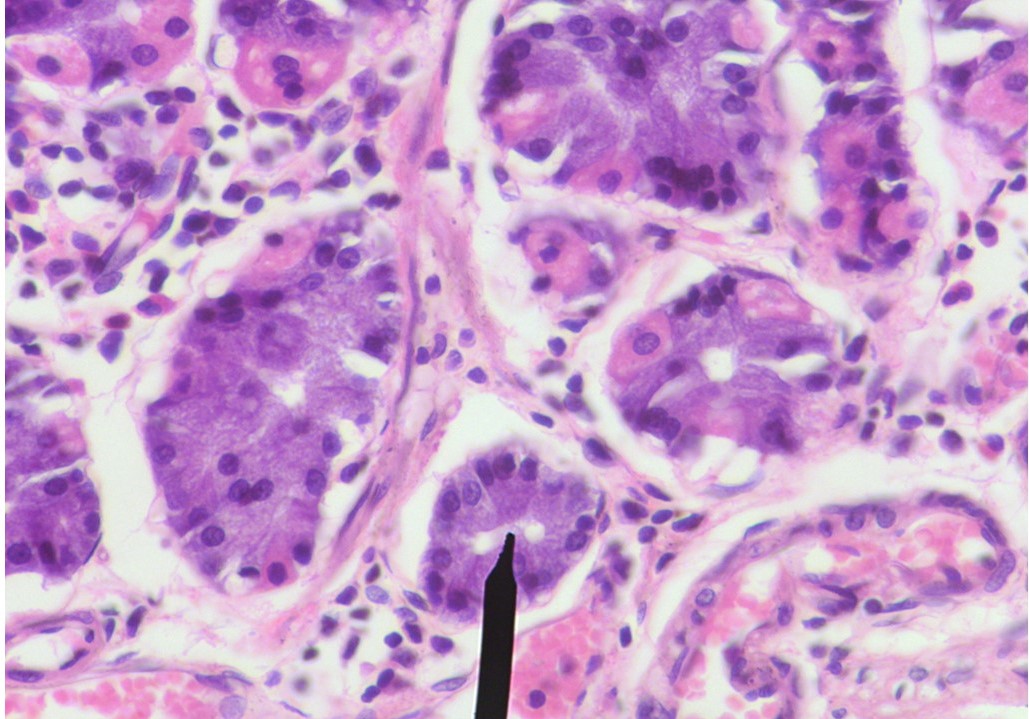
Specialized cells called “chief cells” in the lining of the stomach generally produce digestive enzymes, but when the stomach lining is damaged, these mature, adult cells promptly put on their stem cell hat to multiply rapidly, regenerate damaged tissues and restore homeostatic status quo. The molecular mechanism underpinning this dramatic switch was not known, until now.
A new study in mice led by scientists at the Institute of Molecular Biotechnology of the Austrian Academy of Sciences (IMBA), has now identified a molecular switch for this reserve stem cell state of chief cells. Researchers have found, a genetic switch called p57 is constantly produced in chief cells under normal states but upon injury its expression sharply decreases, followed by the rapid multiplication of chief cells.
“We knew that this behavior must depend on a switch, and we aimed to unveil its precise mechanism of,” said Bon-Kyoung Koo, PhD, an investigator at IMBA and the corresponding author of the study.
The findings were published in the journal Cell Stem Cell on May 5, 2022, in an article titled, ‘p57Kip2 imposes the reserve stem cell state of gastric chief cells’, and could help in developing new treatments for gastric pathologies, such as spasmolytic polypeptide-expressing metaplasia (SPEM), a chronic condition that could lead to cancer.
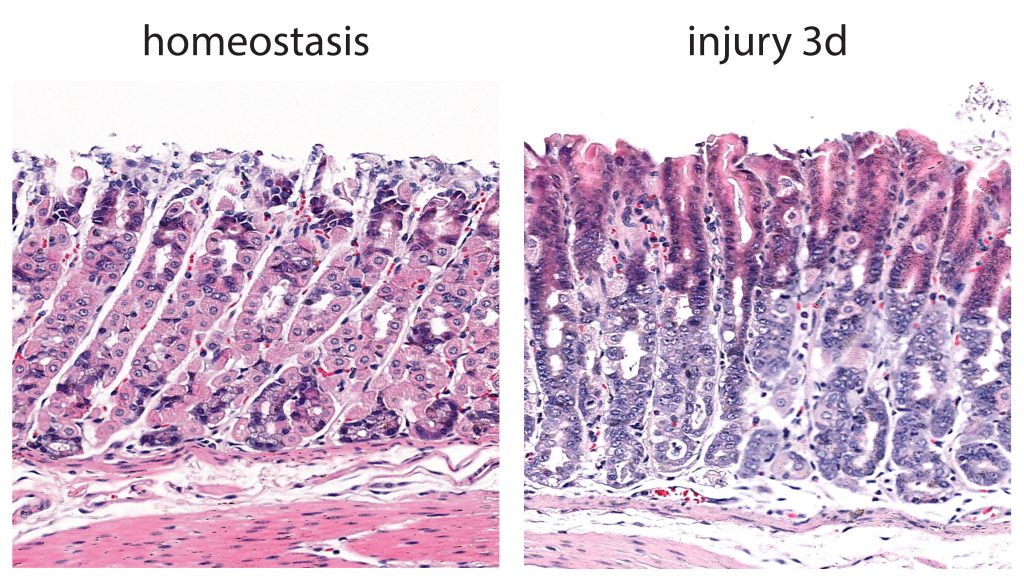
Together with collaborators from Vanderbilt University Medical Center, Nashville, USA, and Pohang University of Science and Technology (POSTECH), Republic of Korea, the scientists developed a mouse model to trace the effects of stomach tissue injury on chief cells. Using single-cell RNA sequencing and doxycycline-induced lineage tracing, the investigators confirmed that the loss of p57 precedes the triggering of proliferation in the chief cell lineage.
“We identified one molecule, p57, as the potential molecular switch we had been looking for,” said Ji-Hyun Lee, PhD, a postdoctoral fellow in Koo’s lab and the lead author of this study. “We confirmed that, upon injury, the level of p57 rapidly decreases in the chief cells and that this is followed by a peak of proliferation within the chief cell lineage.”
In organoids cultured from the main body of the stomach (corpus organoids), the researchers found artificially enhancing p57 expression induced a long-term reserve stem cell state, altered requirements of the immediate surroundings and increased enzyme secretion. This indicated that high levels of p57 in the gastric organoids made the reserve stem cells behave more like mature, secretory chief cells that do not multiply.
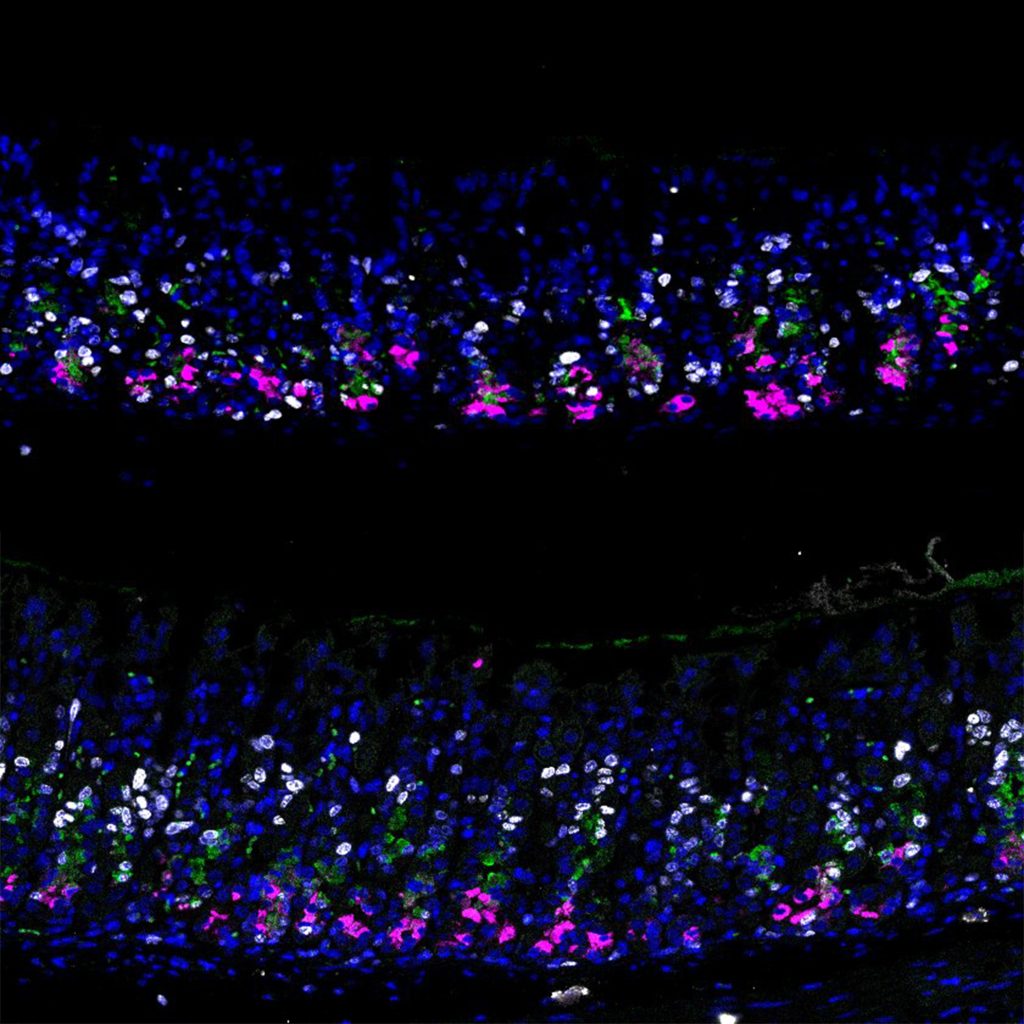
The scientists also found that if they prevented the decrease of p57 expression in chief cells through the induction of constitutive or constant expression of the gene, their ability to multiply and respond to injury was impaired.
“Organoids are usually highly proliferative because of the growth factor cocktail that they need in culture. However, once we introduced p57, the organoids suddenly stopped growing,” said Lee. “In a second step, we reduced the expression of p57, and to our delight, the organoids started proliferating again. This means that the cells did not lose their stemness due to p57. They merely went into a reserve state, exactly mimicking our in vivo observations.”
The team concludes, p57 acts as a gatekeeper that imposes the reserve stem cell state on chief cells when homeostasis is disrupted due to injury.