Researchers at the University of Hong Kong (HKUMed) LKS Faculty of Medicine, and at City University of Hong Kong (CityU) have generated human neural stem cells (hNSCs) that exhibit what they claim is “powerful therapeutic potential” for the treatment of spinal cord injury. The researchers showed that human pluripotent stem cell (hPSC)-derived neural stem cells engineered with reduced expression of a gene called SOX9 differentiate preferentially into spinal motor neurons. When transplanted into a rat model of spinal cord injury (SCI) these neural stem cells generated mature neuronal subtypes, were able to integrate and grow axons that projected over long distances and connected with the recipient’s neurons. Recipients showed reduced glial scarring, and dramatic improvements in locomotor and other functional recovery. The team says their development could pave the way for new therapeutic opportunities.
“Our work reveals a new paradigm in activating the intrinsic programme by using a genetically targeted strategy to enhance the therapeutic potential of hNSCs for treating SCI,” said research lead Martin Cheung Chi-hang, PhD, associate professor, School of Biomedical Sciences, HKUMed. “This approach alters the grafts’ response in the injury environment and confers enhanced neuronal differentiation capacity, survival, and integration, as well as reduced glial scar formation to provide a more effective stem cell therapy for severe traumatic SCI.”
The researchers reported on their developments in Advanced Science, in a paper titled, “Transplanting Human Neural Stem Cells with ≈50% Reduction of SOX9 Gene Dosage Promotes Tissue Repair and Functional Recovery from Severe Spinal Cord Injury,” in which they concluded, “Our findings represent a new paradigm in generating genetically modified hNSCs for the treatment of SCI.”
Traumatic spinal cord injury (SCI results in the progressive loss of neurons involved in motor and sensory functions at and around the site of injury. SCI patients may have permanent paralysis or varying degrees of impairment and loss of sensation, depending on the severity of the injury. Currently, there are no effective treatments for SCI. “The ineffectiveness of current clinical management and treatment regimens can leave SCI patients suffering from lifelong disabilities,” the authors wrote.
![A research team from HKUMed has generated human neural stem cells with powerful therapeutic potential for the treatment of spinal cord injury that paves the way for new therapeutic opportunities. The research team members include: (from left) Wu Ming-hoi, Hui Man-ning, Feng Xianglan, Chen Yong-long, Professor Daisy Shum Kwok-yan, Dr Martin Cheung Chi-hang, Professor Chan Ying-shing, Dr Tam Kin-wai and Amos Lo Lok-hang. A research team from HKUMed has generated human neural stem cells with powerful therapeutic potential for the treatment of spinal cord injury that paves the way for new therapeutic opportunities. The research team members include: (from left) Wu Ming-hoi, Hui Man-ning, Feng Xianglan, Chen Yong-long, Professor Daisy Shum Kwok-yan, Dr Martin Cheung Chi-hang, Professor Chan Ying-shing, Dr Tam Kin-wai and Amos Lo Lok-hang. A research team from HKUMed has generated human neural stem cells with powerful therapeutic potential for the treatment of spinal cord injury that paves the way for new therapeutic opportunities. The research team members include: (from left) Wu Ming-hoi, Hui Man-ning, Feng Xianglan, Chen Yong-long, Professor Daisy Shum Kwok-yan, Dr Martin Cheung Chi-hang, Professor Chan Ying-shing, Dr Tam Kin-wai and Amos Lo Lok-hang. [The University of Hong Kong]](https://www.genengnews.com/wp-content/uploads/2023/08/Low-Res_Group-Photo.jpeg)
The limited locomotor and sensory recovery after SCI has been attributed to the formation of glial scar around the site of injury. “In response to lesion healing, the scar can prevent inflammation from spreading and causing further damage,” the researchers pointed out, but this barrier-like structure also prevents neuronal regeneration. Spinal neurons around the scar cannot be restored due to the low intrinsic regenerative ability of undamaged neurons and the lack of neural stem cells in the adult spinal cord. “… spinal composition and architectures within and around the scar cannot be restored due to low intrinsic regenerative ability of neurons in the adult mammalian central nervous system (CNS) and the postinjury environment.”
Transplantation of human NSCs (hNSCs) derived from human pluripotent stem cells at the SCI sites has been considered a promising therapeutic strategy to compensate for the loss of spinal neurons, and could feasibly enable their connectivity with host neurons, leading to spinal cord recovery. However, the injury environment favours astrocytes instead of neuronal formation which has limited the therapeutic efficacy of grafted hNSCs. “… a hostile microenvironment and deficiency of growth factors in the injured spinal cord limited the therapeutic effects of grafted NSCs that are largely determined by their survival, neurogenic potency, integration capacity, and axial identity,” the researchers continued.
Most transplantation therapies adopt the use of a cocktail of growth factors embedded in the extracellular matrix to enhance the viability and neurogenic potency of grafted hNSCs in SCI rodent models. However, using this approach it takes a long time for hNSCs to mature, and there are “less amount of differentiated neuronal subtypes,” which limits the degree of functional recovery in SCI models. This underlies an urgent need to remodel NSC grafts that can overcome both extrinsic and intrinsic barriers as an effective treatment for traumatic SCI. “Remodelling hNSC grafts to overcome both extrinsic and intrinsic barriers may provide a more effective repair process for traumatic SCI,” they further suggested.
A member of the SOX family of transcription factors, Sex determining region Y-box transcription factor (SOX9) plays a critical role in maintaining NSC multipotentiality, the team continued, and previous work has suggested that persistent expression of SOX9 prevents the de novo neuronal regeneration process in the injured spinal cord. “Functions of SOX9 include maintaining multipotent neural stem properties, promoting astrocyte differentiation, and inhibiting neurogenesis.” This they noted, points to the possibility of targeting SOX9 to generate hNSCs with improved neurogenic potential, and which may better survive the hostile environment for treating SCI.
For their newly reported study the team used shRNA technology to knock down (KD) SOX9 expression in hNSCs (SOX9 KD) cells, in a dose-dependent way.They consistently found that reducing the levels of SOX9 expression in hNSCs by about 50% promoted motor neuron formation, whereas taking SOX9 expression down any lower resulted in compromised cell survival and renewal.
These findings indicate a dose-dependent role for SOX9 in regulating the initiation of neuronal formation, self-renewal, and survival of hNSCs. The team also showed that the enhanced neurogenic potency of hNSCs expressing 50% SOX9 protein was partly attributed to decreased glucose consumption. They detected low glucose uptake and significantly reduced expression of glycolytic genes in cultured SOX9 KD cells, “implying that SOX9 is required for the induction and/or maintenance of high glycolytic metabolism in hNSCs. Interestingly, the scientists further noted, “Recent studies have revealed that neuronal differentiation or/and regeneration requires metabolic remodeling from glycolysis to oxidative phosphorylation. It has been shown that neuronal differentiation from hNSCs requires decreased aerobic glycolysis, whereas constitutive activation of glycolytic genes in hNSCs resulted in robust astrocytes formation and apoptosis of neurons.
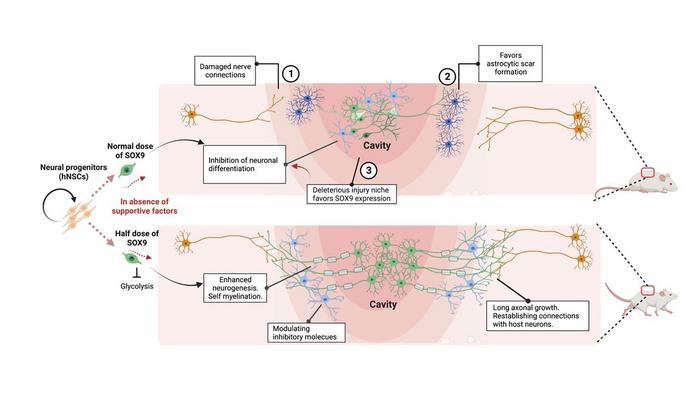
In vivo tests confirmed that the neurogenic and metabolic properties of the SOX9-reduced hNSCs were retained in the cells following transplantation at the site of contusive SCI in rats, and without the need for growth-factor enriched matrices. This suggests that the injury environment did not affect the metabolic state and neuronal differentiation potential of the hNSCs.
SOX9 KD grafts in recipient SCI rats were broadly distributed across the lesion site. And importantly, the authors continued, the grafts exhibited excellent integration properties, predominantly differentiated into motor neurons, and reduced glial scar matrix accumulation to facilitate long-distance axon growth and neuronal connectivity with the host as well as dramatically improve locomotor and somatosensory function in recipient animals.” These results demonstrate that hNSCs with half SOX9 gene dosage can overcome extrinsic and intrinsic barriers, representing a powerful therapeutic potential for transplantation treatments for SCI.”
They further concluded, “Our work reveals a new paradigm in activating the intrinsic program by using a genetically targeted strategy to enhance the therapeutic potential of transplanted hNSCs for treating SCI,” the investigators concluded. “This approach alters the grafts’ response in the injury niche and confers enhanced differentiation capacity, survival, and integration, as well as reduced glial scar formation to provide a more effective stem cell therapy for severe traumatic SCI.”
The team says that to enable the clinical translational of their findings, future efforts should focus on developing genetic strategies to reduce the level of SOX9 activity or expression in hNSCs by ~ 50% for transplantation treatment of SCI patients.



