A study in mice, led by scientists at the Centro Nacional de Investigaciones Cardiovasculares (CNIC), has found that a component of breast milk provides an essential signal that triggers the maturation of heart metabolism in the neonate after birth, allowing the newborn heart to function correctly and ensuring postnatal survival.
The study showed that the fatty acid (FA) gamma-linolenic acid (GLA) maternal milk binds to the retinoid X receptor (RXR) found on the heart cells—cardiomyocytes—triggering maturation of the cells’ mitochondria so that they utilize lipids to generate ATP. Pups bred to lack RXR on their heart cells, or those fed with milk the contained no GLA, died shortly after birth.
The results could have significant therapeutic implications for cardiovascular disorders involving mitochondrial and metabolic dysfunction, as well as for diseases related to alterations in postnatal developmental processes, suggested study lead Mercedes Ricote, PhD, who heads the Nuclear Receptor Signaling group at the CNIC.
Ricote and the international team of researchers reported on their findings in Nature, in a paper titled “γ-Linolenic acid in maternal milk drives cardiac metabolic maturation,” in which they stated, Our results reinforce the emerging idea that mother–infant interactions in early life are major drivers of organismal physiology and highlight the importance of maternal milk ingestion for mitochondrial maturation of perinatal hearts, a finding with major implications for cardiac health.”

The mammalian heart needs a continuous supply of energy to maintain contraction, and cardiomyocytes exhibit what the authors termed a “highly flexible metabolism” that means they can consume a broad range of substrates, including glucose, lipids, lactate and amino acids, to generate the ATP (adenosine triphosphate) that is used as the essential energy currency of the cell.
Fetal cardiomyocytes rely primarily on glucose and lactate oxidation, but after birth, the main source of ATP production is mitochondrial lipid oxidation. Although this process is crucial for the survival of the organism, scientists have known little about the signals that trigger the physiological adaptation of the heart after birth.
“The cardiac fetal-to-neonatal switch is believed to occur progressively during the first two weeks of life, culminating in a functional mitochondrial compartment in which fatty acids are efficiently oxidized by β-oxidation7 (FAO),” the investigators explained. But while this adaptive step is critical for maintaining heartbeat and survival, “very little is known about the molecular mechanisms and upstream signals that instruct this metabolic transition.”
The new study in mice has now found that GLA in maternal milk binds to the retinoid X receptor (RXR) found on the neonatal cardiomyocytes, triggering maturation of the cells’ mitochondria. RXR acts as a nutritional sensor of lipids and vitamin A derivatives, altering gene expression and influencing biological functions such as immunity, cell differentiation, and metabolism. The study found that, once activated by maternal GLA, RXR initiates genetic programs that equip the cells’ mitochondria with the enzymes and other proteins they need to start consuming lipids, the primary source of energy in the mature heart.
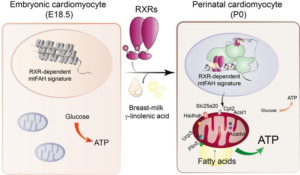
Studies showed that in mouse models in which the RXR genes were deleted in the embryonic heart (embryonic double-knockout; EDKO animals), the lack of RXR in the heart cells prevented mitochondria in the hearts of newborn animals from producing energy correctly, leading to severe heart failure and death shortly after birth. “… 80% of EDKO pups died during the first 24 h of life, and no EDKO newborn survived beyond day 7 after birth,” the team wrote.
Similarly, wild type neonatal mice suckled with milk from mothers that were fed a fat free diet (FFD) also died within 48 hours of birth. The combined results from their studies indicated that milk-FA support metabolic adaptation in the neonatal heart, “… and suggest that activation of a milk-FA–RXR axis is a relevant mechanism for sustaining perinatal life,” the investigators stated.
Subsequent in vitro tests identified GLA as the ligand for RXR, and further experiments in mice showed that newborns from mothers on a fat free diet thrived when their milk was supplemented with GLA. Notably, pups delivered by mothers that were fed a normal chow diet also died when sucked on a FFD milk, “supporting the role of maternal milk, and not lipid deposits during pregnancy, as the relevant GLA source for ensuring perinatal survival,” the investigators further noted.
The study’s combined findings demonstrate that the fatty acid GLA, found in breast milk, is the key signal that ensures correct cardiac function after birth. GLA activates the cell protein RXR, which then directs coordinated gene expression changes to ensure that cardiomyocyte mitochondria mature so that they can produce energy in the extrauterine environment.
“The need to maintain a constant and uninterrupted beat places an immense energy demand on the heart”, explained Ricote. “To meet their energy needs, cardiomyocytes maintain a tight control over the cellular pathways that produce energy. Any imbalance in these bioenergetic mechanisms can lead to the development of serious cardiovascular pathologies.”
For Ricote, part of the study’s novelty “lies in demonstrating that RXR plays a critical role in cardiac muscle, contrary to what was previously thought. This is an important conceptual advance in the field of nuclear receptors.”
According to first author Ana Paredes, PhD, the study presents a new framework for understanding the postnatal adaptations that occur in newborn mammals to meet the requirements of the extrauterine environment. “Birth is a physiological challenge for the newborn,” Paredes explained. “With this study, we show that maternal milk, besides its nutritional function, plays a signaling role by instructing cardiomyocytes that they need to activate their metabolism because they are no longer supported by maternal physiology.”
The results open the way to treatments to modulate RXR activity in cardiomyocytes with specific drugs, including some that already have FDA approval for cancer treatment. “Our study proposes RXR as a possible therapeutic target for neonatal heart disorders and systemic diseases triggered by metabolic errors,” concluded Ricote. And from “a nutritional standpoint,” the team commented, “low GLA abundance in human maternal milk has been linked to growth deficits in newborns, suggesting a potential role of this fatty acid in human neonatal physiology.”



