Mustafa Djamgoz’s interest in bioelectricity began with the first-hand experience of electricity coursing through his own biology. As a teenager growing up on the island of Cyprus, Djamgoz and a friend were building a radio transmitter when he received his first-ever electric shock.
“I was absolutely fascinated,” he said. “What the hell is going on in my body?”
Djamgoz would go on to build a career based on answering that question, earning first a PhD in biophysics and later becoming a professor of neurobiology, studying the ways cells generate bioelectric potentials through ion pumps and channels, utilizing bioelectricity to communicate.
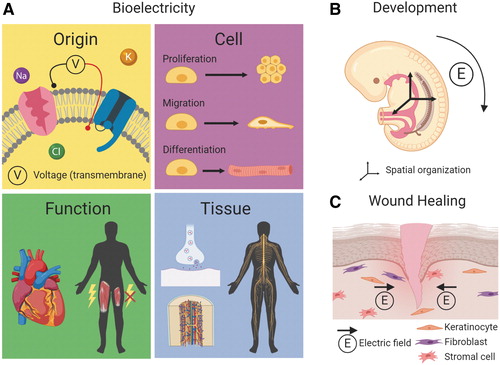
And not just neurons. It’s now appreciated that bioelectric properties are important in all cells and that these properties can shift in response to, or be shifted to treat, the presence of disease.

Today, Djamgoz is professor emeritus of cancer biology at Imperial College London, studying the bioelectric properties of malignant tumors. He’s also the editor of the relatively new Mary Anne Liebert journal Bioelectricity, which was launched in 2018 to provide a focal point for the eponymous, growing, and multidisciplinary field. Recent issues of the journal contain papers on everything from cancer to tissue engineering to cellular aging.
“Bioelectricity has tremendous potential,” according to Djamgoz. “A lot of people, especially on the medical side, don’t quite understand why bioelectricity is of such medical importance. We really want to put it on the map.”
The neuroscience of cancer
Much oncology research focuses on the genetic markers of cancer and the changes in gene expression which drive cancer formation and metastasis. But while genetics remain an essential part of the cancer story, Djmagoz’s research has shown that it’s not the whole story. The bioelectric properties of tumors may be just as important as genetics.
It was back in the mid-1990s that Djamgoz first developed what he would later call the cellular excitability or “CELEX” the “CELEX” hypothesis of cancer metastasis. He identified voltage-gated sodium ion channels in many types of epithelial cancer cells that did not belong, and which led to cancer cells producing action potentials as if they were neurons or cardiomyocytes.
“These are normally inert tissues in your gut or skin,” Djamgoz pointed out. “They become hyperactive, excitable, invasive, antisocial, and it is this electrical excitability that drives the cancer cells into an invasive mode.”
The electrical excitability of cancer cells escaped notice for years because traditional methods of measurement, patch clamp, or microelectrode recordings, lacked the sensitivity to capture the signals.
“Instead of poking the cells with micro-electrodes, we want to plate the cells in a petri-dish with these gold microelectrode arrays on the bottom,” Djamgoz continued. “They are now sitting on these electrodes and buzzing with action potentials!”
Not only do cancer cells become unexpected generators of bioelectricity, but they also communicate with the nervous system, seemingly feeding off of it. In a paper published June 2020 in Biochimica et Biophysica Acta — Reviews on Cancer, Djamgoz showed that sympathetic nervous system stimulation seems to drive the early stages of cancer proliferation, while parasympathetic input drives invasiveness and metastasis.
In a paper in Bioelectricity, Djamgoz reviewed some of the technologies that can potentially influence the bioelectric properties and innervation of tumors to mitigate cancer proliferation and metastasis. This includes electromagnetic “tumor treating fields” already in use to treat glioblastoma, nanoparticles, and implanted coils to stimulate the vagus nerve, and the repurposing of old drugs such as beta-blockers and sodium channel blockers as anti-cancer agents.
Djamgoz has started his own company to pursue some of these potential treatments, CELEX Oncology, and believes clinical applications—even if they begin by making existing therapies better and less toxic—are not that far away.
“We’re talking probably the next five years,” he says, “something like that.”
Bioengineering with bioelectricity
Bioengineers tend to focus on biomechanical and chemical properties of cells and biomaterials, according to Kent Leach PhD, professor of bioengineering at the University of California, Davis. From 2000 to 2019, for instance, there were 10 times as many bioengineering publications examining mechanical and chemical cues than bioelectrical cues.
But while properties such as stiffness, viscoelasticity, and material degradation are important for bioengineered materials, “the capacity to capture the benefits of electrical stimulation in more biocompatible materials is very interesting and a relatively new area,” Leach says.
In a paper in Bioelectricity, Leach and his colleagues review the use of bioelectrical signaling as a blueprint for tissue engineering, exploring how endogenous electrical signals and conductive substrate materials can be used to direct cell differential and promote bioengineered tissue functionality, and how the field must progress to take advantage of this new dimension.
“We are eager to develop conductive, biocompatible materials to test their efficacy in models of bone regeneration, muscle regeneration, and even skin repair,” Leach says. “The majority of evidence suggests that cells are responsive to these materials even without external electrical stimulation, suggesting there is an exciting opportunity to leverage these materials for many applications that we have not yet considered.”
The paper reviews a number of the biocompatible materials that have been explored for bioengineering in different tissue systems, such as Polypyrrole (PPY), “the most studied conductive polymer for biomedical applications.” But the selection of conductive biomaterials remains difficult, Leach says, in that materials used must not harm neighboring cells when they degrade, and researchers don’t yet have great control over conductivity in the more biocompatible materials.
“The understanding of fundamentals is lacking because we typically avoid electrical stimulation in hydrated tissues,” he continues. “After all, what’s the best way to throw the toaster into the bathtub?”
Bone fractures and bioelectric fields
Rather than studying the bioelectric properties of many materials and tissues, Swee-Hin Teo, PhD, and his team at Nanyang Technological University in Singapore are focused on understanding the role of electromagnetic fields in healing bone tissue.
“Bone is a piezoelectric material,” Teoh says, generating bioelectric potentials in response to mechanical loading. “In our work, we hypothesized initially that we must treat bone as an electrical conductor as well.”
The conductive properties of bone suggest the possibility of bioelectric interventions in cases of trauma, and in a paper in Bioelectricity titled “Effects of Pulsed Electromagnetic Field Intensity on Mesenchymal Stem Cells,” Teoh and his colleagues described the potential for, and parameters around, the use of pulsed electromagnetic fields to improve bone regeneration techniques.
The current gold standard treatment for bone injuries, such as the hip fractures common in older adults, is an invasive autologous bone graft. Pulsed Electromagnetic Fields (PEMFs) represent an alternate mode of therapy and have been shown to encourage bone growth, but there’s little consensus on the optimal PEMF parameters.
To begin to constrain those parameters, Teoh and his colleagues measured the effects of varying PEMF intensities on the proliferation, differentiation and mineralization of mesenchymal stem cells taken from rabbit bone marrow. Exposing cultured stem cells to 50 Hz pulsed magnetic fields varying in intensity from 0.2 to 0.9 mT for 30 minutes a day for 21 days, they found 0.6 mT field strength the most successful at increasing cell proliferation, “showing tremendous potential to enhance bone healing in the first two weeks post-fracture.”
The time constraint of 21 days is also an important finding, Dr. Teoh, as “previously we would have thought there is no window of efficacy.”
It may be possible to influence other stages of bone healing—such as cell differentiation and mineralization—with different PEMF parameters, Teoh says, but that will require further research.
“We are also looking at the effects of PEMF on embryonic development and bone formation using chicken embryo models,” he says. “It would be interesting to see how PEMF could affect not only bone healing, but the early formation of bone itself.”
Bioelectrical properties of aging
Until fairly recently, many of the experiments in bioelectricity were fragmented among different research areas, according to Tailise Carolina de Souza-Guerreiro, PhD. “It is only recently that bioelectricity researchers came together and started building a field,” she says.
It is within that new interdisciplinary framework that scientists are exploring how electrophysiology is regulating myriad cellular processes, including the focus of de Souza-Guerreio’s work as a postdoctoral researcher at the University of Warwick: mitochondrial function and aging.
In a Bioelectricity paper written with her colleague, University of Warwick Associate Professor of Biology Munehiro Asally, PhD, de Souza-Guerreio reviewed insights in aging gained from studies of mitochondrial electrophysiology in yeast. “Many of the modulators for vertebrate lifespans, such as calorie restriction,” they write, “are conserved from yeast to humans.”
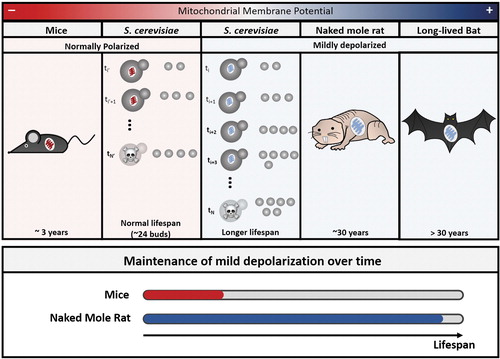
“This new framework is changing how researchers are thinking about aging and mitochondria,” de Souza-Guerreio said, and leading to new questions. “What is the threshold between the beneficial mitochondrial depolarization and the deleterious energy-depleting effects?”
Future studies on chemical compounds and longevity should screen candidate compounds for their effects on mitochondrial electrophysiology as well as other markers of aging, according to de Souza-Guerreiro, as part of a truly interdisciplinary approach.
“I believe a multidisciplinary investigation is needed to understand the biological, biophysical, and chemical aspects of mitochondrial electrophysiology,” she says, “to guide us and pave the path for future anti-aging pharmaceutical interventions targeting mitochondria.”
Emerging bioelectric cancer medicines
Frankie Rawson, PhD, a research fellow in the University of Nottingham school of pharmacy, agrees with de Souza-Guerreiro on the importance of multidisciplinary investigation in bioelectricity and sees the construction of large teams to be one of the defining challenges for the field.
“We understand that electricity is important in biology. How can we sense it and manipulate it for the treatment of disease?” he says. “You need multidisciplinary insight and tools to really do that.”
Rawson’s own interdisciplinary work began with preclinical drug toxicology in animals, moved to developing nano-electrode sensors to test toxicity in non-animal models, and from there led to his applying that sensor technology to the measurement of both ionic and faradaic currents in cancers, a relatively new discipline. “Our tools to sense [bioelectricity] are poor,” he says. “We really don’t have great tools to measure, for example, sub-cellar electrical effects.”
In a paper published in Advanced Therapeutics titled “Toward hijacking bioelectricity in cancer to develop new bioelectric medicine,” Rawson and his colleagues examine the electrical properties of tumors and argue that for the field to move forward, researchers need to take a more holistic view of cancer bioelectricity.
“We need to stop looking at single ionic currents,” and take a more systems biology perspective to bioelectricity, he says. “You probably need to understand it from a global, cellular perspective, and interfere with multiple electrical relays.”
It’s a view not altogether dissimilar to Djamgoz’s, but Rawson believes that when it comes to application, bioelectric medicine cannot rely on techniques such as surgically implanted coils for nerve stimulation. If it is to become mainstream “It’s got to be done wireless,” he says. “We can think about building technology to more carefully target specific bioelectrical relays that then gives rise to our ability to either prevent cancer cells from proliferating or kill them.”
Rawson is also researching the use of injected nanoparticles in conjunction with external electric fields. “When we apply external fields, we modulate the chemistry on the particle, which directs the cells to kill themselves,” he says.
Like other researchers in the field, Rawson acknowledges just how much remains to be learned about bioelectricity but is also bullish on the prospects for bioelectric medicine in the clinic.
“The field of bioelectric medicine is already worth billions of dollars and it’s expected to increase significantly,” he says. “We are now at a stage where it’s not mainstream yet, but certainly it’s starting to shift that way.”


