Scientists have recognized the relationship between normal organ development and cancer for more than 100 years. More recently, researches have begun to emphasize that the heterogeneous nature of most cancers largely contributes towards their clinical response. Now, a team of investigators at Huntsman Cancer Institute (HCI) at the University of Utah (U of U) and the Salk Institute for Biological Studies, have created the first single cell resolution atlas of genes that control the formation of breast tissue. The atlas provides a comprehensive molecular map that the researchers are optimistic will be used to help understand how breast cancers form and to pinpoint new ways to prevent, diagnose, and treat the disease.
Findings from the new study were published recently in Cell Reports through an article titled “Single-Cell Transcriptomes Distinguish Stem Cell State Changes and Lineage Specification Programs in Early Mammary Gland Development.”
“We know that cancers are driven by mutations in specific genes that cause cells to multiply or to survive where they don't belong,” explains senior study investigator Benjamin Spike, Ph.D., an HCI cancer researcher and assistant professor of oncological sciences at the U of U. “During this process, cancer cells misappropriate whole circuitries of cell biology that are important and normal at other times in development. Our team works to understand this process by researching how cells normally work to form tissues. We also identify developmental programs that have been taken over by cancers. Some of these programs should make good targets for treating or preventing disease, even if they are not directly mutated.”
Until recently, tools didn't exist that could simultaneously and individually analyze all of the different types of cells in an organ and their active genes, the segments of cellular DNA that encode functional molecules such as proteins.
“We addressed this significant knowledge gap by generating a single-cell transcriptome atlas encompassing embryonic, postnatal, and adult mouse mammary development,” the authors wrote.
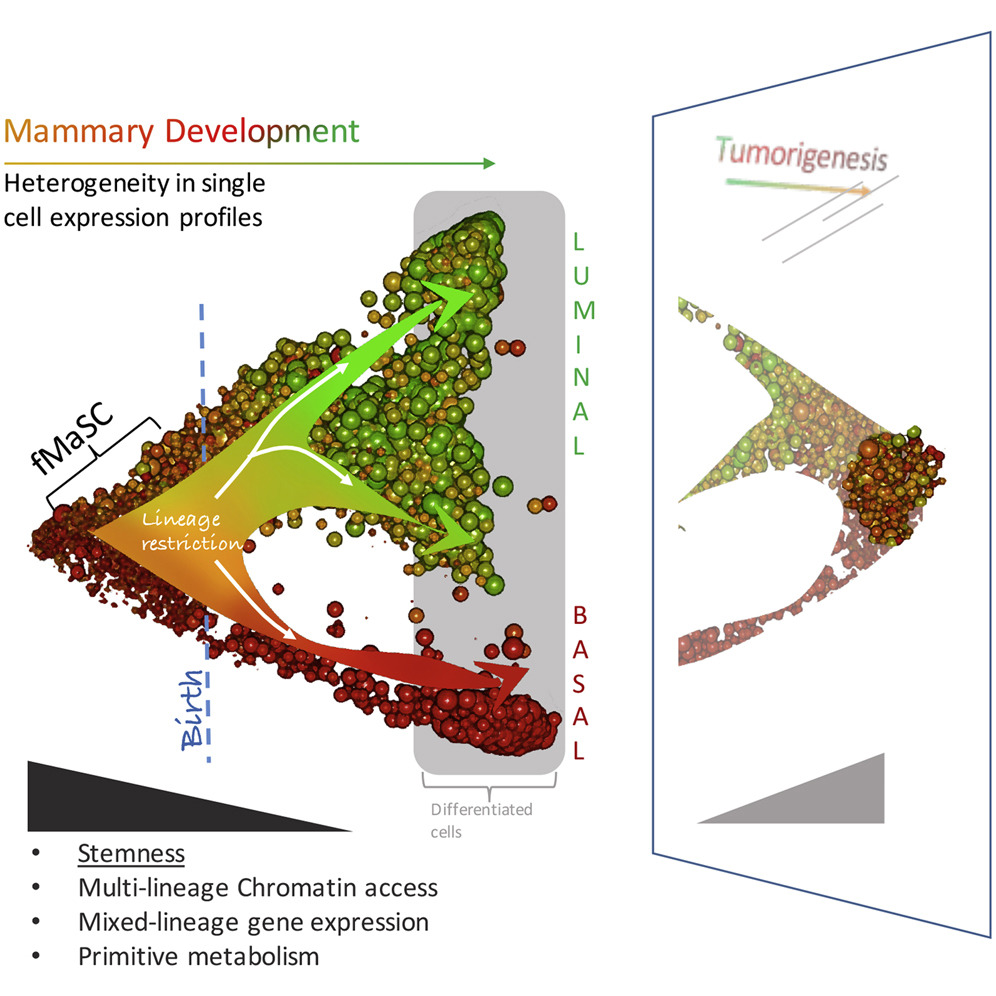
In the current study, the research team isolated breast cells from mice during various stages of breast tissue development. The researchers then used new genome sequencing technology to analyze which genes are turned on and off in more than 6,000 individual cells during normal development. This approach revealed a list of hundreds of potentially critical genes for further study as regulators of normal and cancerous development. These genes may provide targets for certain breast cancers that don't have molecular targeted therapies.
“From these data, we mapped the chronology of transcriptionally and epigenetically distinct cell states and distinguished fetal mammary stem cells (fMaSCs) from their precursors and progeny,” the authors penned “fMaSCs show balanced co-expression of factors associated with discrete adult lineages and a metabolic gene signature that subsides during maturation but reemerges in some human breast cancers and metastases.”
The data from this study provides new insights into the organizing principles of tissues and the molecular definitions of the cell types comprising them. Moreover, the research shows that breast cells in early development that are not fully differentiated into mature cell types activate more genes, resulting in mixed profiles and expression of some genes in unexpected breast cell types.
“This research promises new targets for these cancers, including genes involved in specialized cellular metabolism that are shared between early breast development and certain breast cancers,” Dr. Spike concludes.