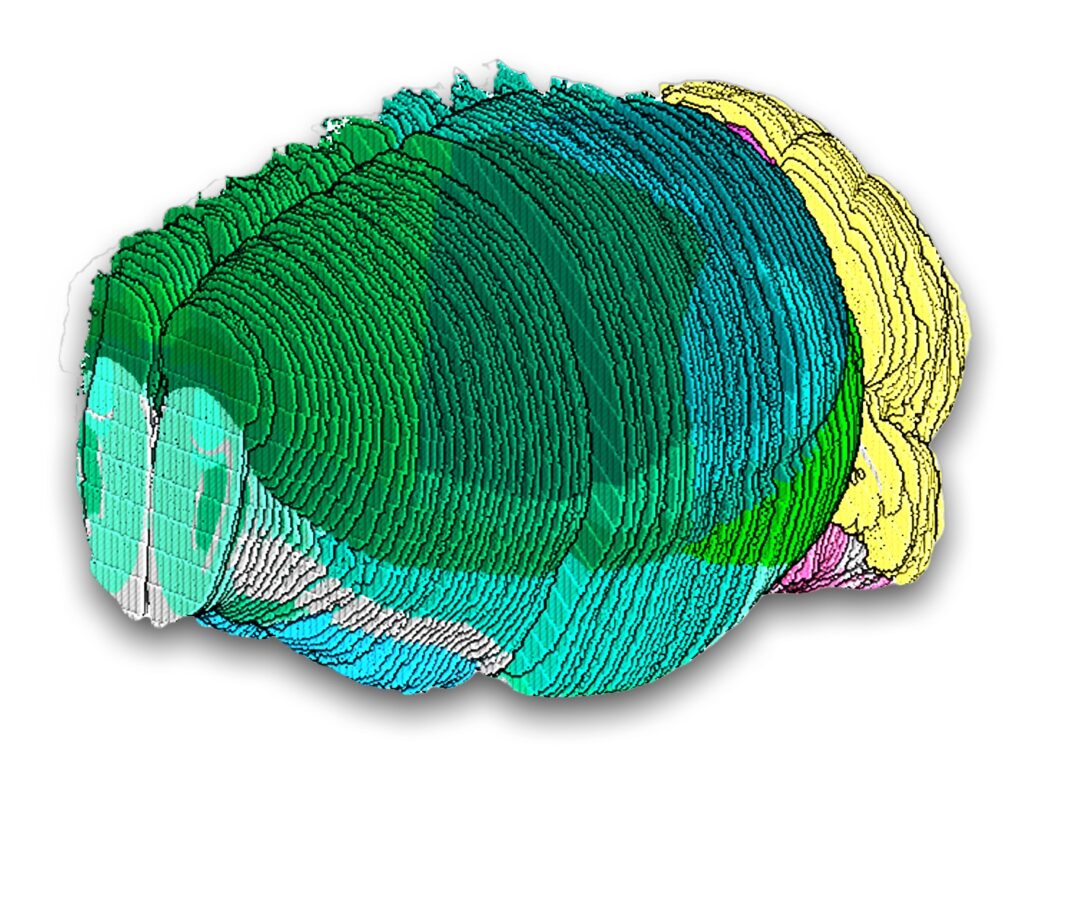
A comprehensive map has been built of cell types in a mammalian brain using spatial and single-cell genomics. The atlas delineates several thousand cell populations across the entire mouse brain, revealing surprising cellular diversity in understudied brain regions and offering deeper views of brain structures than were possible before.
The work is published in Nature, in the paper, “The molecular cytoarchitecture of the adult mouse brain.”
The effort was led by Evan Macosko, MD, PhD, an institute member at the Broad and associate professor and attending psychiatrist at Massachusetts General Hospital, and Fei Chen, PhD, a core institute member at the Broad and an assistant professor in the department of stem cell and regenerative biology at Harvard University.
The team’s analysis identified several thousand unique cell populations—an estimated 90% of all cell populations in the mouse brain. The scientists found most cellular diversity within relatively understudied subcortical areas of the brain, especially the midbrain, pons, medulla, and hypothalamus.
“We suspected the most diversity would be found in these areas, so we prioritized them in our profiling,” said Macosko. “A lot of the real nuts and bolts stuff that a brain is doing is in these basic areas, which have received very little attention compared to the cortex. Our results underscore the need to study them more deeply.” The researchers also discovered clues about cellular function and the potential roles of brain structures in disease.
“Efforts like these generate crucial resources for the neuroscience community because the brain is so enormously complicated,” said Chen. The researchers created an online browser to house and share their datasets with the scientific community (www.BrainCellData.org/).
The team analyzed the full transcriptome of cells from nearly 100 regions across the mouse brain using high-throughput single-nucleus RNA sequencing, the preferred approach for efforts to create a human brain atlas. This resulted in more than four million profiles of gene activity, which they clustered into nearly 5,000 unique cell populations, most of which were neuronal cells.
The team next applied Slide-seq—their spatial technology. They transferred 101 serial sections, spanning the volume of a single mouse brain. They sequenced those transcripts and aligned that spatial data to an existing 3D reference atlas, enabling them to assign each transcript to a known brain structure, representing more than 1.7 million mapped cells. They then combined detailed and well-sampled cell type profiles from the single-nucleus sequencing dataset to locate each cell type in each slice to generate a detailed and thorough atlas of the entire mouse brain. They also analyzed the dataset to come up with two or three marker genes that could be used to uniquely identify almost all of the cell populations.
The team also revealed how neurotransmitters are used by different cell types in various brain regions. In addition, they demonstrated their atlas’s utility in revealing where disease-associated genes are active, for example, specific neuronal cells that are enriched for expression of genetic factors associated with schizophrenia.
The authors said that other scientific groups can use the transcriptomic profiles, spatial localizations, and sets of marker genes they identified to study particular cells of interest. Groups may also use the data by integrating it with their own more detailed examination of a particular brain region. “We hope that our atlas can both empower the community in their own work and allow them to further explore the cell types we identified,” said Jonah Langlieb, a computational biologist in the Macosko lab who led the work along with Nina Sachdev.