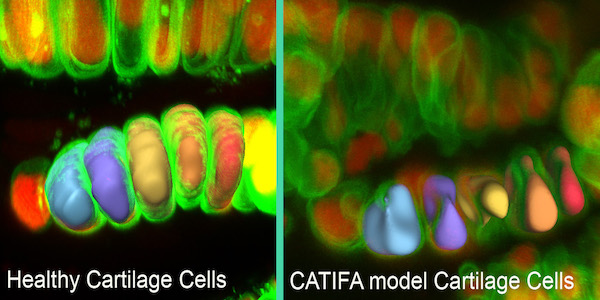
Cartilage cells in the zebrafish model of the disease CATIFA (right) have reduced volumes and irregular shapes compared to healthy cartilage cells (left) because of a defect in collagen secretion. [Ela Knapik, M.D.]
The linking of genotypes with phenotypes remains a difficult gap to narrow in genetic medicine. Connecting that information to specific human diseases is even more challenging. Now, by combining three sources of phenotypic data, a team from Vanderbilt University Medical Center has pointed out a role for collagen secretion in a wide variety of clinical symptoms, resulting in a newly identified genetic syndrome.

Ela Knapik, MD, associate professor of medicine at Vanderbilt University Medical Center, and her colleagues, combined three sources of information: a zebrafish model, a pediatric genetic disease, and a database of electronic health records linked to a DNA biobank to discover the syndrome caused by mutation of a single gene. They named it CATIFA, an acronym for its core symptoms: cleft palate, cataracts, tooth abnormality, intellectual disability, facial dysmorphism, and ADHD.
The work is published in Nature Medicine in a paper titled “Phenome-based approach identifies RIC1-linked Mendelian syndrome through zebrafish models, biobank associations and clinical studies.”
“Each of these three data sources has its own advantages and shortcomings for disease discovery,” said Knapik. “I thought, why don’t we use them concurrently.”
Knapik and her team were exploring the function of a gene called ric1 in zebrafish. Using a zebrafish genetic screen, the team identified an essential role for the ric1 gene in skeletal biology. They knew that mutation of ric1 disrupted collagen secretion and caused craniofacial and other skeletal defects in fish.
They learned that another group of investigators had reported a mutation in the RIC1 gene in multiple children from a single family who had pediatric cataracts. Knapik’s team evaluated the zebrafish for cataracts, but didn’t find any. The story might have ended there, but Knapik was intrigued. Did the children have other symptoms that hadn’t been characterized because their most pressing problem was cataracts? What might those symptoms be?
To uncover additional symptoms, Knapik and her colleagues turned to BioVU, Vanderbilt’s DNA biobank and database of de-identified electronic health records.
Eric Gamazon, PhD, research instructor in medicine, and Nancy Cox, PhD, the Mary Phillips Edmonds Gray professor of genetics and director of the Vanderbilt Genetics Institute, had previously developed a computational method, called PrediXcan, to correlate genetically regulated gene expression with patient phenome—the clinical characteristics included in the electronic health record. When applied to BioVU, the method generates a list of clinical characteristics linked to reduced expression of a specific gene.
Using a gene-based phenome-wide association study (PheWAS) in the EHR-linked BioVU biobank, they show an association of reduced expression of RIC1 and musculoskeletal and dental conditions. More specifically, the team described that “whole-exome sequencing identified individuals homozygous-by-descent for a rare variant in RIC1 and, through a guided clinical re-evaluation, it was discovered that they share signs with the BioVU-associated phenome.”
The list they generated for reduced RIC1 expression matched characteristics Knapik and her team observed in the fish, she said.
The match of phenotypes was an “aha moment” Knapik noted. She recalled that it was “unreal” and she “couldn’t stop smiling” when she heard the result. She knew that pediatric patients were the next logical step. “I knew at that moment that we needed more information about the patients with RIC1 mutation and pediatric cataracts.”
The team in Saudi Arabia that had reported the patients with RIC1 mutation agreed to reevaluate those patients for the BioVU-derived list of symptoms. Knapik said the investigators were surprised to find the constellation of symptoms in their patients: eight children from two related families.
The symptoms of the new disease, CATIFA, can be explained by loss of collagen function, Knapik said. Collagen is the main structural component of the extracellular matrix—the “mortar” between the cellular “bricks.”
Using zebrafish, Knapik and her team were able to determine that the RIC1 protein is part of the cellular machinery that processes and ships collagen out of the cell.
“In the absence of RIC1, you don’t get the collagen shipment, and you don’t have a matrix,” Knapik said. “That leads to the broad spectrum of symptoms that we find in the electronic health records of adults and in the children with CATIFA.”
Knowing the mechanistic underpinning for the disease and the symptoms that may arise will improve and personalize care for children with CATIFA and for adults with similar symptoms caused by less efficient activity of RIC1, Knapik added. The study suggests a new paradigm for accelerating the discovery process for disease mechanisms, Knapik said.
“With knowledge coming from animal models, human common disease biobanks, and rare Mendelian diseases, we can put together a complete picture of gene function and advance toward better diagnosis, treatment, and prevention,” she said.