An international research team has used high tech methods to uncover a mechanism through which the right combination of bacteria in the gut can lead to microbiome resilience to dietary alterations, and changes in brain function. Studies in fruit flies, headed by scientists at Champalimaud Centre for the Unknown, in Lisbon, working with collaborators at the University of Glasgow, demonstrated how two gut bacteria establish metabolic cross-feeding that enables them to grow when the fruit fly diet lacks essential nutrients, and that this also changes host decision-making and can improve reproduction.
“We found that the two bacteria exchange metabolites and that this cross-feeding (syntrophy) enables them to grow and act on the animal even if diets lack the nutrients that are essential for them,” explained Darshan Dhakan, PhD, a postdoctoral researcher and co-author of the team’s published paper in Nature Communications. “Specifically, we now understand that Lactobacillus strains produce lactate which is used by the Acetobacter strains to synthesize amino acids and other metabolites, Dhakan said. “These are then used by the Lactobacillus strain which cannot synthesize them to continue to produce lactate. Furthermore, these bacterial amino acids are very likely used by the animal for egg production. But most importantly, we now understand that the lactate is also used by the Acetobacter bacteria to change the behavior of the fly.”
Dhakan and colleagues reported on their findings in a paper titled, “Metabolic cross-feeding in imbalanced diets allows but microbes to improve reproduction and alter host behavior.”
A balanced intake of essential amino acids (eAAs) is crucial to ensure the wellbeing and health of all animals. The essential amino acids are the building blocks of proteins but they also influence how much offspring animals produce, and what animals decide to eat. “Given the importance of a balanced dietary intake of AAs, organisms are able to direct their feeding choices to homeostatically compensate both for the lack and over-ingestion of AAs,” the authors wrote. In female Drosophila melanogaster fruit flies, for example, both AA deprivation and mating induce changes in specific neuronal circuits that impact on food choice, they explained. “Remarkably, the removal of any of the 10 eAAs from the fly diet is sufficient to induce this strong protein appetite.”
Scientists have already demonstrated how the gut microbiome can modulate host physiology and behavior. “As such, gut bacteria have also been shown to influence feeding behavior, food choice, and reproduction,” the investigators continued. Intriguingly, the team at the Champalimaud Centre for the Unknown had previously shown that the microbiome plays an important role in dictating how amino acids affect the brain. What was most puzzling was that bacteria could only affect the decisions of the animal when they were present in specific combinations. But why different types of bacteria are needed to influence brain function and alter host physiology isn’t known. This is the puzzle that principal investigator and senior author Carlos Ribeiro, PhD, and his team set out to tackle, using the fruit fly as a model. “To study how bacteria affect their host physiology is a daunting task in organisms with very complex microbiomes,” commented Sílvia Henriques, PhD, postdoctoral researcher and co-author of the published study. “This is where the fly and its less complex microbiome emerges as a powerful tool. It allows us to precisely dissect the mechanisms used by the microbiota to change the host’s feeding decisions.”
The Ribeiro lab team had previously discovered that flies deprived of single essential amino acids develop a strong appetite for protein-rich foods. However, in flies that were associated with two bacteria, Acetobacter pomorum (Ap) and Lactobacillus plantarum (Lp), which are very abundant in the microbiome, their preference for protein was drastically reduced and the flies preferred to eat sugar. “Interestingly, the association of flies with any of these bacteria alone could not reduce yeast appetite,” Ribeiro said. “Thus, in this new study, our main focus was to understand why these two particular bacteria need to be present to change the feeding behavior of the fly.”
Research by several groups working on the microbiome, including the Ribeiro Lab, has shown that it is typically necessary for a community of bacteria, rather than isolated bacteria, to have an impact on host behavior—and this was most likely due to specific metabolites that the bacteria produce. The team set out to measure the metabolic interactions established between the bacteria in the fruit fly microbiome, and to map how specific bacteria and their metabolites affect the animal.
To follow the feeding choices of the flies, researchers took advantage of a sensor developed in the lab—the flyPAD—and used it to measure in detail the feeding pattern of individual flies. Then they used bacterial mutants to understand the impact of specific functions of the bacteria, on host behavior. And with collaborators at the University of Glasgow, the researchers also harnessed a sophisticated technique called isotope-resolved metabolomics, which enabled them to track the metabolites that were exchanged between the two different bacteria.
They found that the presence of Lp bacteria stimulates Ap bacteria to produce and excrete isoleucine (Ile)—which is an essential AA for Lp, and can be used by Lp to grow in the absence of isoleucine in the fly’s diet, the investigators wrote. They further found that it was the lactate produced by Lp that was the primary contributor of this bacterium to the mutualistic relationship, and that it was this that changed the fly’s feeding behavior away from protein appetite.
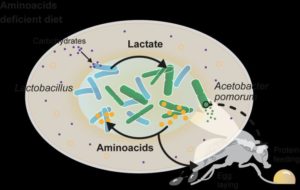
“As such, it is possible to substitute Lp with lactate and observe the same level of protein appetite suppression, showing that this metabolite is necessary and sufficient for modifying protein appetite in the presence of Ap,” they noted. “Here we use an interdisciplinary approach including isotope-resolved metabolomics to show that in Drosophila melanogaster, Acetobacter pomorum, and Lactobacillus plantarum, a syntrophic relationship is established to overcome detrimental host diets and identify Ap as the bacterium altering the host’s feeding decisions,” they wrote. “Overall our data are in agreement with a model, in which Lp and Ap grow as a community in the fly, where they engage in a syntrophic interaction buffering them from adverse host dietary conditions. Lp provides lactate to Ap, which it uses to synthesize and secrete AAs. This ensures Lp growth, even in detrimental dietary conditions in which limiting AAs are missing, as well as an increase in egg production in malnourished flies.”
By establishing this cross-feeding relation, the bacterial community becomes resilient to drastic dietary changes, and can continue to grow in the intestines of animals that ingest diets that lack nutrients that are essential to the survival of particular microbiome species survival. “These metabolic interactions within the Ap/Lp community allow these two bacteria to create a “circular economy,” in which they both optimally use the available nutritional resources provided by the host diet, allowing them both to overcome detrimental host diets and boosting their metabolic output,” the team commented.
Ribeiro further noted, “It is well established that our diet affects both the microbiome and our brain. What makes it complicated is the microbiome then, in turn, affects how diet affects us and what animals decide to eat. This makes it a very complex puzzle to solve. But by combining the right technologies with the right experimental system we can get at the heart of the mechanisms by which the microbiome interacts with our diet to affect our brain and our body. Importantly we show that the right associations of bacteria can make the microbiome resilient to dietary perturbations explaining why some animals and people might be more sensitive to the nutrient content of food than others. It is also a beautiful example of how nature establishes circular economies where nothing gets wasted and everybody gains.”
The team suggests that the research represents an important example of how model organisms can be used to disentangle the influence of diet on the microbiome, and to understand the individual contributions of gut bacterial species on brain function and behavior. “The methodologies that were used in this study will allow us to identify all the metabolic interactions established amongst bacteria and will allow us to understand the precise mechanisms responsible for altering what animals decide to eat and brain function. Those insights can then be used to guide the search for similar mechanisms in animals with much more complex microbiomes, including in humans,” concluded Ribeiro.



