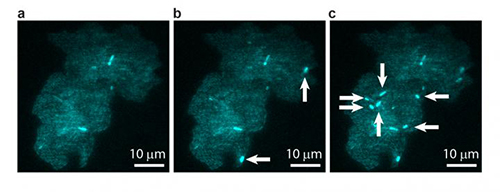
![A bacterial colony showing individual cells undergoing transposable element events, resulting in blue fluorescence. Images are shown at (a) t = 0, (b) t = 40 min, and (c) t = 60 min, with arrows indicating newly occurring events in each image. [T.E. Kuhlman, University of Illinois at Urbana-Champaign, with permission from PNAS]](https://genengnews.com/wp-content/uploads/2018/08/Jun14_2016_UnivIllinois_BacterialColony1541491839-1.jpg)
A bacterial colony showing individual cells undergoing transposable element events, resulting in blue fluorescence. Images are shown at (a) t = 0, (b) t = 40 min, and (c) t = 60 min, with arrows indicating newly occurring events in each image. [T.E. Kuhlman, University of Illinois at Urbana-Champaign, with permission from PNAS]
While jumping genes may seem to hop pell-mell through the genomes of host cells, potentially triggering transcriptional and mutational surprises, they betray at least one kind of regularity—the rate at which they leap to and fro. This rate does not fluctuate at random. Rather, it seems to depend on the host cell’s growth and environmental factors.
This result emerged from studies conducted at the University of Illinois at Urbana–Champaign, where scientists were dissatisfied with existing methods for studying jumping genes, or transposable elements (TEs). Rather than rely on these methods, which are bulk techniques that average results from multiple cells, the scientists developed a fluorescent protein–based reporting system that allowed them to focus on individual cells. That way, instead of having to make do with time and cell ensemble averages, the researchers could capture cell-to-cell variation and temporal variability in individual cells. In short, the scientists found a way to observe jumping genes in action directly, in real time.
The scientists, who were led by physics professors Thomas Kuhlman and Nigel Goldenfeld, published their findings June 13 in the Proceedings of the National Academy of Sciences, in an article entitled, “Real-Time Transposable Element Activity in Individual Live Cells.” The article detailed how the use of a simple plasmid-borne TE allowed the scientists to observe surprisingly rich jumping-gene activity.
“We find that TE activity depends upon the TE’s orientation in the genome and the amount of transposase protein in the cell,” wrote the authors of the PNAS study. “We also find that TE activity is highly variable throughout the lifetime of the cell. Upon entering stationary phase, TE activity increases in cells hereditarily predisposed to TE activity.”
To observe individual TE events in living cells, the scientists devised a synthetic biological system based on the bacterium Escherichia coli. The scientists coupled the expression of fluorescent reporters—genes that encode (in this case, blue and yellow) fluorescent proteins—to the jumping activity of the transposons. The scientists could then visually record the transposon activity using fluorescent microscopy.
“In this study, we were able to see that there is actually more jumping gene action going on than might have been expected from previous studies,” said Dr. Kuhlman, whose team performed the in vivo experiments. “What's more, we learned that the rates at which these genes jump depend sensitively on how the cells are growing—if there is food available for the cells to grow, for example. In other words, jumping gene activation isn't entirely random, it's dependent on environmental feedback.”
“These are genes that hop around and change location within the genome of a cell,” Dr. Kuhlman continued. “We hooked that activity up to a molecular system, such that when they start hopping around, the whole cell fluoresces. In our experiment, cells fluoresced most when they weren't very happy. One school of thought suggests that an increased mutation rate like this might be an advantage in such unhappy conditions, for cells to diversify.”
To help design the experiment and to calculate what would have happened if the jumping occurred in a purely random fashion, Dr. Goldenfeld's team developed computer simulations of the growth of bacterial colonies and predicted what the experimental signal would look like in the random case. These calculations showed that the experiments could not simply be interpreted as random transposon activity and even provided clues as to sources of nonrandomness, including environmental feedback and heredity.
“Our work involved a good deal of computational image analysis, followed by statistical analysis,” explained Dr. Goldenfeld. “To extract signals and conclusions from the raw data, simulation and theoretical calculations were integral to the experimental design and interpretation.”
“The over-arching long-term research goal is a deeper understanding of how evolution works at the molecular level,” concluded Dr. Kuhlman. “Direct observation of how genomes in cells restructure themselves allows for a precise determination of adaptation rates and may shed light on a host of important evolutionary questions, ranging from the emergence of life to the spread of cancer, where cells undergo rapid mutations and transformations of their genomes.”