A targeted delivery system boosts the numbers of anti-inflammatory immune cells within the brain to restrict brain inflammation and damage. This therapeutic method, which delivers interleukin 2 to the brain, harnesses the body’s immune system to protect against brain cell death following brain injury, stroke, and in a model of multiple sclerosis.
The research is published in Nature Immunology in the paper, “Astrocyte-targeted gene delivery of interleukin 2 specifically increases brain-resident regulatory T cell numbers and protects against pathological neuroinflammation.”
A leading cause of traumatic brain injury is the inflammatory response to the injury; swelling of the brain can cause permanent damage. While inflammation in other parts of the body can be addressed therapeutically, the blood-brain barrier creates a challenge as it prevents common anti-inflammatory molecules from getting to the site of trauma.
The recent identification and characterization of a small population of regulatory T cells resident in the brain, the authors noted, present a potential therapeutic target.
“Our bodies have their own anti-inflammatory response, regulatory T cells, which have the ability to sense inflammation and produce a cocktail of natural anti-inflammatories,” noted Adrian Liston, PhD, a senior group leader at the Babraham Institute—a partner organization of the University of Cambridge in the U.K. “Unfortunately, there are very few of these regulatory T cells in the brain, so they are overwhelmed by the inflammation following an injury. We sought to design a new therapeutic to boost the population of regulatory T cells in the brain so that they could manage inflammation and reduce the damage caused by traumatic injury.”
In this study, the team found that regulatory T cell numbers were low in the brain because of a limited supply of interleukin 2. Together the team devised a new approach that allows more interleukin 2 to be made by brain cells, thereby creating the conditions needed by regulatory T cells to survive. A gene delivery system based on an engineered adeno-associated viral vector (AAV) was used: this system can cross an intact blood-brain barrier and deliver the DNA needed for the brain to produce more interleukin 2.
Matthew Holt, PhD, visiting group leader at VIB-KU Leuven Center for Brain & Disease Research said, ”For years, the blood-brain barrier has seemed like an insurmountable hurdle to the efficient delivery of biologics to the brain. Our work, using the latest in viral vector technology, proves that this is no longer the case; in fact, it is possible that under certain circumstances, the blood-brain barrier may actually prove to be therapeutically beneficial, serving to prevent ‘leak’ of therapeutics into the rest of the body.”
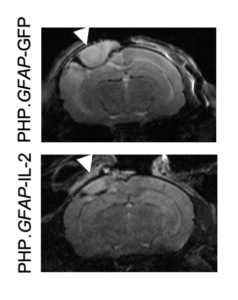
“Seeing the brains of the mice after the first experiment was a ‘eureka moment’—we could immediately see that the treatment reduced the size of the injury lesion,” noted Lidia Yshii, PhD, senior scientist at the KU Leuven and VIB.

“By understanding and manipulating the immune response in the brain, we were able to develop a gene delivery system for IL2 as a potential treatment for neuroinflammation. With tens of millions of people affected every year, and few treatment options, this has real potential to help people in need. We hope that this system will soon enter clinical trials, essential to test whether the treatment also works in patients,” said Liston.



