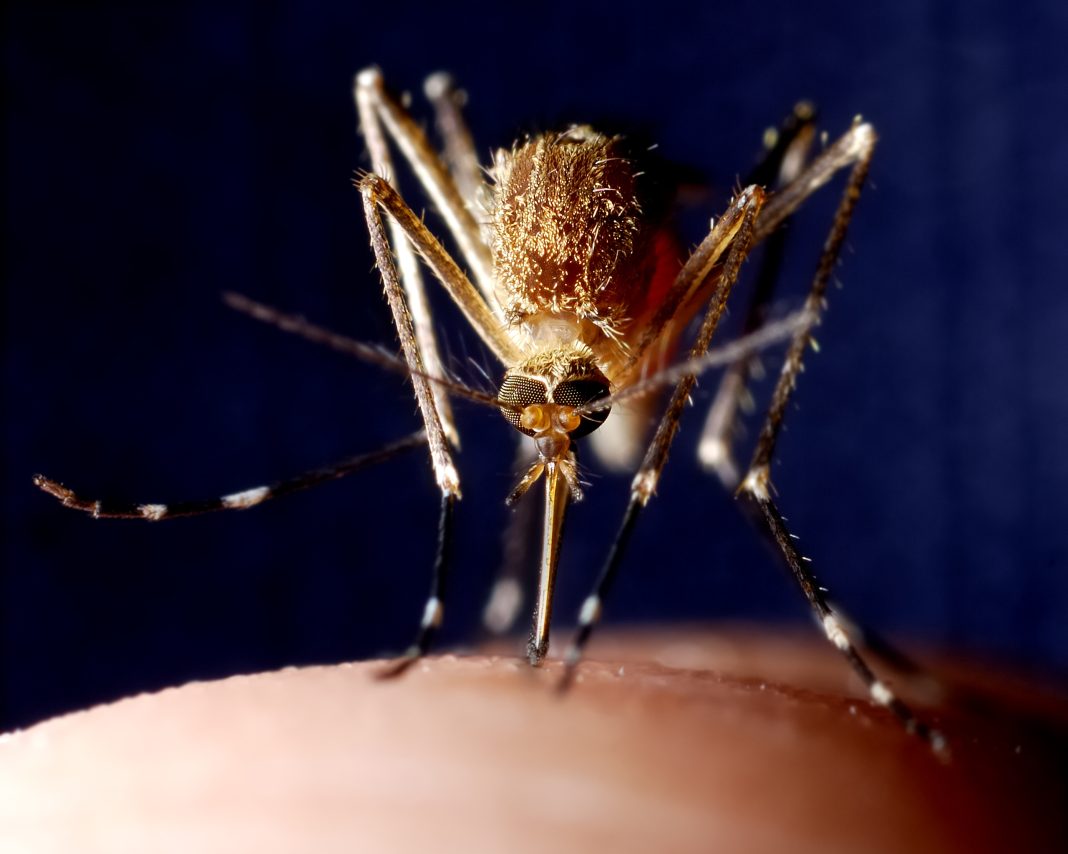
A widespread bacteria called Wolbachia pipientis and a virus that it carries can cause sterility in male insects by hijacking their sperm, preventing them from fertilizing eggs of females that do not have the same combination of bacteria and virus. A new study led by microbiome researchers at Penn State has now uncovered how this microbial combination manipulates sperm, altering long noncoding RNA (lncRNA) and DNA during sperm development to establish a paternal-effect embryonic lethality known as cytoplasmic incompatibility (CI). The results, the team suggests, could potentially help scientists devise refined techniques to control populations of agricultural pests and insects that carry diseases like Zika and dengue to humans.
“Wolbachia is the most widespread bacteria in animals and lives symbiotically within the reproductive tissues of about 50% of insect species, including some mosquitos and flies,” said research co-lead Seth Bordenstein, PhD, professor of biology and entomology, director of the One Health Microbiome Center at Penn State. “Wolbachia has genes from a virus called prophage WO integrated into its genome. These genes—cifA and cifB—allow the bacteria to remarkably manipulate sperm and quickly spread through an insect population for their own good.”
The team reported on their findings in Science, in a paper titled “Prophage proteins alter long noncoding RNA and DNA of developing sperm to induce a paternal-effect lethality.” In their paper, the team noted, “This study identifies prophage endonucleases modulating eukaryotic lncRNA and DNA that play crucial roles in animal reproduction. A cascade of early modifications established prefertilization in the testes ultimately results in postfertilization paternal-effect embryonic lethality.”
A prophage-containing bacterial symbiont, W. pipientis, inhabits the reproductive tract of approximately half of all arthropod species worldwide and “… selfishly alters host reproduction to increase the relative number of symbiotic females that transmit the bacteria to the next generation,” the authors wrote. When a male and female insect that both have Wolbachia mate, they successfully reproduce and pass on the bacteria. But when a male with Wolbachia mates with a female that doesn’t carry the bacterium, the sperm are rendered lethal to the fertilized eggs, which is known as CI. This system cunningly increases the proportion of offspring with Wolbachia and the virus in the next generation, because females with the bacteria successfully reproduce more frequently than females without. “Specifically, CI results in embryonic death when symbiotic males mate with aposymbiotic females,” the team further explained. “Nullification of death and thus, rescue of CI, occurs when transmitting females and their eggs harbor the same strain of Wolbachia.”
This system is being used in several ongoing pilot studies across the world to control insect pests and the harmful viral diseases they carry. For example, to control a population of agricultural or human pests that do not have the Wolbachia bacteria, scientists release males that do carry the microorganism.
“This shape change is incredibly important to the success of sperm, and any interference can impact the sperm’s ability to travel in the female reproductive tract and successfully fertilize the egg,” said research co-lead Rupinder Kaur, PhD, assistant research professor of biology and entomology at Penn State. “The transition is highly conserved in almost everything from insects to humans. Defects in this process can also cause male sterility in humans.”
According to the researchers, sperm is particularly prone to DNA damage and repair during this transition. Through their studies they found that sperm exposed to Wolbachia, or to the Cif proteins alone, had an elevated level of DNA damage at this stage. The DNA damage, if not repaired in a timely fashion, can result in abnormal sperm genome packaging, male infertility, and embryonic inviability.
“These results confirmed the impact of Wolbachia and Cif proteins at this stage of sperm development, but we still wanted to know what was happening at earlier stages to trigger these changes,” Kaur said. “We conducted a series of tests to explore the structure and biochemical function of the Cif proteins and found that they can cleave messenger molecules called long noncoding RNA, which sets the stage to interfere with downstream development and function of the sperm.”
The researchers used the D. melanogaster with Wolbachia model to test the potential link between the bacteria and long noncoding RNA. “lncRNAs predominantly transcribed in Drosophila testes play a crucial role in chromatin organization during sperm development and male fertility,” the investigators pointed out. They found that Wolbachia—or the Cif proteins alone—reduced the amount of these RNAs. Additionally, mutant flies with reduced expression of these RNAs in conjunction with Wolbachia had elevated levels of embryonic inviability because it augmented the defective transition process of sperm development. So, Kaur explained, the virus proteins control sperm by depleting the long noncoding RNAs required for a normal sperm function. The Cif protein-related errors in sperm development “… correlated with postfertilization embryonic DNA damage associated with CI,” the team wrote.
“Long noncoding RNAs do not make any proteins themselves, but they can have profound impacts on regulating the function of other genes required for sperm development,” Bordenstein said. “By altering this noncoding part of the genome, we found that Cif proteins start impacting sperm right from the earliest stages of development. Wolbachia’s prophage WO genes act like master puppeteers, manipulating sperm development in a way that allows their genes and the symbiotic bacteria to quickly spread through arthropod populations.”
In their paper, the authors concluded, “The discovery that intranuclear Cifs alter central-dogma features of sperm biology, spanning noncoding RNA depletion and DNA damage, reveals a molecular tangled bank of tripartite phage endosymbiont-animal interactions that are vital to animal gametogenesis and embryogenesis.”
Because the process of sperm development looks similar across the animal kingdom, the researchers said that knowledge of this process could lend insight into sterility challenges in humans as well as inform new control methods of harmful insect populations. “Hence, prophage proteins interact with eukaryotic macromolecules during gametogenesis to create a symbiosis that is fundamental to insect evolution and vector control,” they noted.
“One of Wolbachia’s superpowers is that it blocks pathogenic RNA viruses such as Zika, dengue, and chikungunya virus, so mosquitos with Wolbachia do not pass these viruses on to people when they bite,” Bordenstein said. “So, releases of both male and female mosquitos with Wolbachia in an area where it isn’t already present leads to replacement of the population with mosquitos that can no longer pass on a viral disease. The World Mosquito Program is now using Wolbachia to control viruses in 11 countries. With this study, we reveal the underlying mechanics of how this process works so we can fine-tune the technique to expand its scope in vector control measures.”
“Now that we have reverse engineered this process, we can fine-tune methods of population control with Wolbachia that are already in use,” Kaur said. “We plan to take advantage of this knowledge to augment currently existing disease vector and pest control methods, and perhaps emulate the technique without Wolbachia or virus proteins in the long-term.”