The Global Synthetic Biology Summit, otherwise known as SynBioBeta, kicked off in San Francisco this week with an opening talk by Kevin Ness, PhD, CEO of Inscripta. The relatively young genome editing company has, until now, been best known for MAD7—their Cas9-alternative nuclease for genome editing. But that is likely to change as Ness’s talk titled “A New Era in Genome Engineering” launched a benchtop instrument for genome-scale engineering.
The Onyx, as the instrument is named, is an all-in-one genome editing platform that gives scientists the ability to create libraries of millions of precisely engineered single cells in one experiment. According to Ness, it requires only two things from the researcher: a hypothesis to validate and an endpoint. Inscripta, Ness claims, will take care of the rest.
“CRISPR-based gene editing technology,” Ness notes, “has the potential to transform our ability to precisely engineer genomes, but it has not yet fully delivered on this promise due to significant limitations.” The reason, Ness explains, is that researchers are restricted to making single, simple edits. They can choose to scale up either the number of locations in the genome to edit, or the complexity in the type of mutations—but not both. Up until now, Ness says, trying to draw genotype to phenotype associations based on the genome has been like being “in a dark room with a laser pointer.”

“We have to sample a larger space than ever before,” Ness notes, driving that point home in his talk with an exercise in underscoring the complexity of the genome—noting that the number of possible genetic permutations in the yeast genome is larger than the number of atoms in the universe. “We need new approaches,” he adds.
Enter the Onyx. It is a fully automated workflow that includes software, hardware, chemistry, and assays that enables massively parallel, trackable editing of single cells. It “looks like nothing in your lab because it does something nothing in your lab has ever done,” notes Jason Gammack, chief commercial officer at Inscripta.
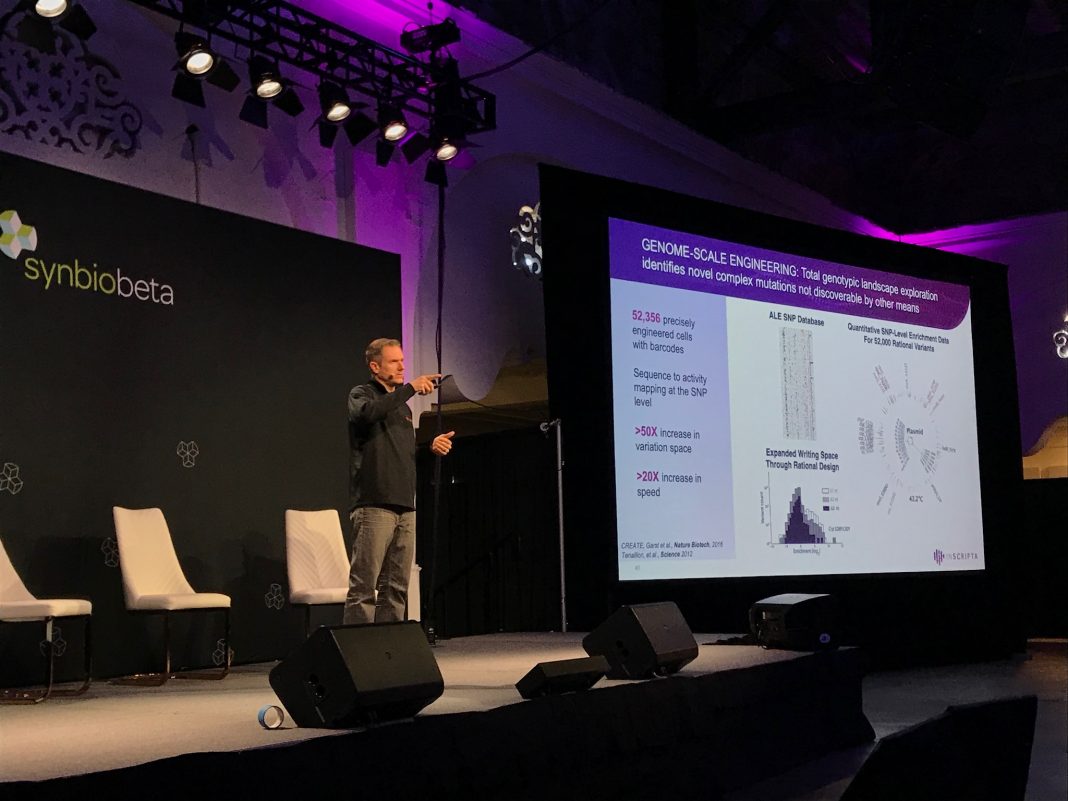
Details of the platform’s inner workings flew under the radar in Ness’s talk, with assertions of the importance of microfluidic modules to handle the cells and that Inscripta’s chemistry is “key.” At the heart of the platform’s ability to scale the number of edits is the “CRISPR-enabled trackable genome engineering” or CREATE method of covalently attaching each guide RNA to homologous repair cassettes. Barcoded libraries are built that allow each cell to be tracked by the proprietary microfluidic system.

Chris Voigt, PhD, professor of advanced biotechnology in the department of biological engineering at MIT, was an early user of the Onyx who found the platform useful to advance his research. Voigt, a synthetic biologist, sought to produce linalool—the compound that lends the hoppy flavor to beer—in bacteria. But E. coli find linalool production toxic. By harnessing Inscripta’s vast collection of genome edited E. coli strains that are resistant to a variety of environmental insults (the resistome), Voigt identified linalool-resistant bacterial strains that could serve as linalool producers.
Jim Collins, PhD, professor of medical engineering and science at MIT was another early user of the system. Ness recalled approaching Collins immediately after he heard Collins’s talk at synbiobeta two years ago, to tell him about Inscripta’s work. Collins went on to use the platform to investigate novel antibiotic resistance pathways, noting that the technology allowed them “to explore the genetic dependencies of antibiotic function in unprecedented detail.” Collins adds that the technology “has significant potential to lead to the development of novel therapeutics against our most difficult antibiotic-resistant pathogens.”
The Onyx platform, Ness asserts, overcomes these limitations and will give all scientists the power to design, engineer, evaluate, and track results of powerful genome engineering experiments in their own lab. And, he hopes that this technology will transform the capabilities of genome writing and enable researchers to focus their efforts on testing multiple biological hypotheses in parallel without having to outsource their experiments or spend precious time and resources on the laborious task of optimizing complex gene editing methods.
Inscripta’s goal, Ness tells GEN, is to turn genome biology from an observational science to an interventional science. He explains that CRISPR’s potential might be “special”, but it simply has not delivered. Inscripta wants to “unlock researchers from these constraints” and let them access the “full, rich flavor” of the genome.
It remains unclear, at this early stage, whose constraints will be unlocked and if the Onyx will have a presence in labs beyond biotech companies and large institutes like the Broad Institute. CRISPR genome editing is being done very efficiently, and inexpensively, at a large scale, in labs all over the country. George Church’s lab at Harvard Medical School has already surpassed 13,000 gene edits in a single cell. And, the Onyx is certain to come with a hefty price tag. But, as Ness told GEN in June, “Inscripta will do for genome writing what Illumina did for genome reading.” If his prediction is correct, Onyx-based large-scale genome editing, like next-gen sequencing, may open up a new revolution in understanding the genome.