Delivery services often boast of their speed. For example, Federal Express is credited with the slogan, “When there is no tomorrow.” Yet speed shouldn’t eclipse safety—even if safety issues tend to be addressed in the fine print of delivery contracts. To see a delivery service that makes safety as prominent as speed, look at Viral Express. It’s not a company. It’s an appropriation of nature that represents the delivery vehicle of choice in gene therapy.
The safe delivery of genetic cargo has preoccupied gene therapy for at least two decades, going back to gene therapy’s earliest clinical trials. In 1999, during an early trial evaluating a gene therapy against the rare disorder ornithine transcarbamylase deficiency, a teenaged patient, Jesse Gelsinger, suffered a fatal immune reaction to a viral vector. This tragic outcome caused a temporary halt to the field of gene therapy.
There are multiple viruses that can be used to deliver genes to cells, and each virus has its advantages and disadvantages. Viruses that receive consideration include lentiviruses, but adeno-associated viruses select AAVs) are becoming the workhorses of gene therapy.
Although Gelsinger’s trial made use of an adenovirus, closely related AAVs are inspiring optimism. They have many desirable features. They are nonpathogenic, they can infect a lot of different and notoriously hard-to-reach tissues, and they do not integrate into the genomes of target cells. Consequently, AAVs are driving a resurgence of gene therapy. Already, AAVs are being used to deliver two FDA-approved gene therapy products.
Pioneering AAV-based gene therapies
“Ten to twenty years ago, there was a big question mark in the field as to whether gene therapy was even going to be a viable approach to treat human disease,” says David V. Schaffer, PhD, professor of chemical engineering at the University of California, Berkeley. “Thankfully, we are past that now.” Schaffer has been in the gene therapy field for 26 years. He notes that it is now quite clear that “as a result of Luxturna and Zolgensma, the field has arrived.”

David V. Schaffer, PhD
Professor of Chemical Engineering
UC Berkeley
Luxturna, which is used to treat people with a biallelic RPE65 mutation–associated retinal dystrophy, was approved by the FDA in December 2017. It was, in fact, the first AAV-based gene therapy approved by the FDA for an inherited disease. Developed by Spark Therapeutics, Luxturna is an AAV2-based gene therapy.
Zolgensma is an AAV9-based gene therapy that replaces the survival motor neuron 1 gene in children with spinal muscular atrophy. Developed by AveXis select which was acquired by Novartis in 2018 union the gene therapy received FDA approval earlier this year.
These two successes suggest that after a two-decade struggle, gene therapy is nearing a breakout point, beyond which many potential treatments become possible. To fully exploit the opportunities ahead, notes Schaffer, gene therapy must deploy gene therapies that can bring enough genetic cargo to targeted tissues. select AAVs can hold roughly 4.7 kb.) Also, payloads need to be delivered, says Schaffer, “in a way that is stealthy [with respect to immune interactions], safe for patients, more efficacious, and more economical for manufacturing.”
The existing clinical successes have been a result of having a clinical target for which the existing versions of AAV are sufficient. But some AAV-based gene therapies could involve an invasive route of administration select like Luxturna) or very high doses of virus select like Zolgensma or some of the Duchenne muscular dystrophy therapies being evaluated in current trials).
Despite such limitations, AAVs are the best bet for future gene therapies. There are many kinds of AAV, and each has different strengths and weaknesses. And researchers across the world are working to improve AAV delivery, both at large companies and academic labs, to advance gene therapy. The task is urgent because for many patients afflicted with genetic diseases, the Federal Express slogan rings all too true.
Evolving the next generation of AAVs
Despite the recent successes, gene therapy still needs to overcome serious challenges—primarily those concerning delivery. Existing AAVs are “good,” but they haven’t been optimized as gene delivery vehicles. They have, Schaffer indicates, evolved for their own purposes and intents within nature. The properties of natural AAVs often differ from the properties AAVs should have if they are to succeed in therapeutic applications. Natural AAVs are not targeted, possess little efficiency with human cell types, and can have interactions with the immune system. Those are three of the properties, Schaffer explains, that need to be addressed when making the next generation of AAVs.
Although Schaffer knows where on the virus to focus on to increase efficiency—the surface amino acids of the viral capsid—he notes that we “don’t necessarily know how to do it.” The ways in which the virus interacts with cells and tissues and fluids are incredibly complex. “We don’t have enough rational knowledge,” Schaffer laments, “about how to make specific amino acid changes to reroute the virus.” To sidestep this difficulty, the Schaffer laboratory follows the same approach that nature used when creating these viruses in the first place—evolution. Actually, the lab uses directed evolution, a deliberate form of natural evolution that earned its pioneer the 2018 Nobel Prize in Chemistry.
In the Schaffer laboratory, directed evolution starts with the diversification of the surface of the virus. To date, the technique has helped the Schaffer laboratory create over a billion variants. These are organized in 36 libraries, each of which contains 10–100 million viruses. The libraries are complex, in part, because they have been elaborated from various AAV starting points. select Some of the libraries have been created around AAVs that, according to bioinformatic ancestral reconstruction, likely existed millions of years ago.) In addition, the libraries reflect how portions of natural serotypes have been stitched together to create random chimeras.
Scientists in the Schaffer laboratory package each viral particle with the DNA that encodes the capsid, barcoding each particle with its own DNA. They then inject the entire library into an animal model and select which viruses make it to the tissue of interest. They do it over again so that the pool of a billion variants converges to a handful of AAVs that can be individually characterized. The selection of the fittest occurs not only in a Darwinian way, but also a high-throughput way.
These procedures formed the basis of 4D Molecular Therapeutics, a company co-founded by Schaffer and David Kirn, MD, in 2013. Schaffer, the CSO, and Kirn, the CEO, have been “running on all cylinders” to get the company’s technology into the clinic.
Balancing specificity and productivity
Eric D. Kelsic, PhD, CEO of Dyno Therapeutics, thinks that using new technologies may open a route that can get AAVs into the clinic more quickly. The company, which was launched in 2018, focuses on developing and applying new technologies to capsid engineering. Kelsic spent several years laying the foundation for Dyno while he worked as a postdoc in the laboratory of George Church at Harvard Medical School. Since last November, the company has been operating at the LabCentral biotech innovation hub in Cambridge, MA.
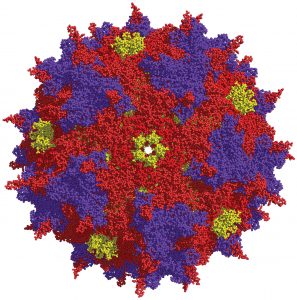
At UC Berkeley, David V. Schaffer, PhD, and colleagues use high-throughput directed evolution approaches to engineer viral vectors. By introducing molecular-level changes to viral capsids, such as the AAV capsid shown here, the scientists hope to improve gene therapy delivery.
Dyno eschews building random libraries, which can pose tradeoffs between diversity and quality. Instead, the company uses a systematic approach to the engineering of new AAV capsids. Dyno indicates that it has developed a workflow that incorporates high-throughput DNA synthesis, DNA sequencing, and machine learning to allow the data-driven design of future libraries.
According to Kelsic, Dyno’s libraries may be “wide” or “deep.” A wide library encompasses all possible single amino acid changes select substitutions, insertions, or deletions) and indicates which individual changes are favorable. Combining these changes can lead to deep libraries, which can be analyzed with machine learning technology to guide the optimization of the capsids.
With conventional methods, making a capsid with increased specificity may result in less productivity. But this tradeoff could be avoided with Dyno’s data-driven design approach. It may be too early to say whether this approach will change the gene therapy game, but Kelsic hopes that his company’s process to optimize AAVs will be a valuable step forward.
AAVs that have the spark
“It’s good that someone is talking about this,” affirms Sandy Macrae, PhD, CEO, Sangamo Therapeutics. “We all get excited about the other stuff, but the concerns surrounding AAVs are critically important.”

Sandy Macrae, PhD, CEO
Sangamo Therapeutics
“AAV6 is our workhorse at the moment,” he points out. The company is also thinking ahead about preparing vectors for projects that will begin in 2020 and 2021.
“The field is moving very quickly,” Macrae observes. He notes that when he became CEO of Sangamo three years ago, the gene therapy field was “very academic.” Back then, a few small companies were preoccupied with R&D work. Now, there are lots of companies engaged in clinical work.
One of those companies, Spark Therapeutics, takes a holistic approach to vector development, with both internal and external efforts to identify better capsids. Internally, the company employs about 80 researchers select roughly a quarter of the total Spark workforce) who work on the development of vectors and the optimization of AAVs for different indications. Spark’s researchers also collaborate with academics in this area, according to the company’s CSO, Federico Mingozzi, PhD.
One area that Spark works to improve is the problematic interaction of AAVs and the immune system. Because AAV is a natural virus to which humans are exposed, Mingozzi explains, there is a large proportion of the population that carries antibodies against AAV—which is disqualifying for entrance into clinical trials.
“We don’t have a good way to enroll a patient who has antibodies that are reactive against the capsid,” admits Mingozzi, who adds that this is not as much of a problem with AAVs that go directly into the eye, brain, or muscle. In addition, 100% of treated subjects develop antibodies when given the vector the first time, making the possibility of a second treatment extremely complicated.

Federico Mingozzi, PhD, CSO
Spark Therapeutics
With immune evasion just one of many AAV properties that Spark is trying to improve, how does Mingozzi commit to utilizing existing AAV vectors when even better vectors might emerge from the pipeline at any time? “You have to find the right compromise between what you have in hand with what may come up soon,” Mingozzi offers. There are, Macrae suggests, other hard questions. He asks, “Is the risk of switching between phases of development overcome by the benefit you would get from a more effective vector?”
Switching vectors between Phases II and II is complicated, Macrae notes. “This field is moving so quickly, it’s tempting [to switch], but [doing so] comes with risks and potential delays to development.”
Mingozzi adds, “If you wait for perfection, you will never do anything.” Sometimes you want to push through a first-generation product that has the chance to be beneficial to patients. As there are new advances, there will be a second generation. He thinks that this scenario is likely to happen with the gene therapies for Duchenne muscular dystrophy. “We see a lot of promising results,” he says, “but there will probably be a next-generation set of products that will work much better.”
“It makes sense,” he asserts, “to stick with one product and bring it all the way.” Mingozzi tells GEN that if you talk to patients, especially those with a lethal disorder, they will say that they want drugs now. “They don’t want you to tweak the drugs until they’re perfect,” he reports. “[They know] their disease will not wait for the timelines of drug development.”
Manufacturing AAVs at Scale
It’s hard to have a conversation about the role of AAVs in gene therapy without mentioning AAV manufacturing, which looks simple enough—just “big vats of viruses,” says Sandy Macrae, PhD, Sangamo Therapeutics’ CEO. He hastens to add, however, that there is a “great science about the whole process.” Manufacturing capacity, he continues, is “a rare resource”—so rare, in fact, that it obliges all companies in gene therapy to plan carefully.
It isn’t a case of having an idea of what you could do to a vector today, and putting it into a patient tomorrow, he explains. Usually, a year separates identifying what you want and having the clinical material in hand, and much of the lag is due to the difficulty of manufacturing a new vector. First, he notes, “you have to make sure that you have somewhere to manufacture it.”
“Three years ago, the field was not thinking about this at all,” notes David V. Schaffer, PhD, professor of chemical engineering, University of California, Berkeley. “If this technology takes off, we are going to be treating more patients with more conditions.” We are reaching a situation, he continues, where manufacturing is a major bottleneck.
Macrae indicates that there is a shortage of manufacturing companies, and their services are in high demand. He adds that there is a shortage of people with process development and manufacturing experience relevant to AAVs.
Some companies, like Spark Therapeutics, may opt to manufacture their own vectors. According to Sparks’ CSO, Federico Mingozzi, PhD, the company has a team devoted to manufacturing. He says that companies smaller and younger than Spark may be more interested in going to contract manufacturing organizations select CMOs) that make AAVs. He warns, however, that if you approach a CMO to make AAVs for a clinical trial today, you may be at the end of a horrendously long waiting line.
AAV-manufacturing companies include Lonza, Brammer Bio select now part of Thermo Fisher Scientific union Paragon Gene Therapy select a unit of Catalent Biologics union the Chinese company WuXi Advanced Therapies, Novasep, Vigene Biosciences, and Virovek.
Some companies are offering innovative solutions to get around the bottleneck problem. GE Healthcare Life Sciences, for example, developed an offering called Enterprise Solutions that is “an end-to-end biomanufacturing product and services solution,” says Florence Vicaire, the company’s global gene therapy business development leader. She tells GEN that GE Healthcare delivers a fully qualified manufacturing site. According to Vicaire, the company’s concept, called KUBio, includes “the walls as well as the equipment that goes inside” that will enable the customer to grow, harvest and purify the viral vector. The customer can also opt to buy just the single-use manufacturing equipment, which GE calls the FlexFactory.
GE Healthcare can deliver a fully qualified manufacturing facility in 12 to 18 months. Each facility is designed to be scalable and adaptable to accommodate the customers’ needs, whether the customer is at a clinical or commercial production stage. At this moment, when gene therapy’s greatest challenge is manufacturing, innovative solutions like this may make a big difference in getting products from bench to bedside.



