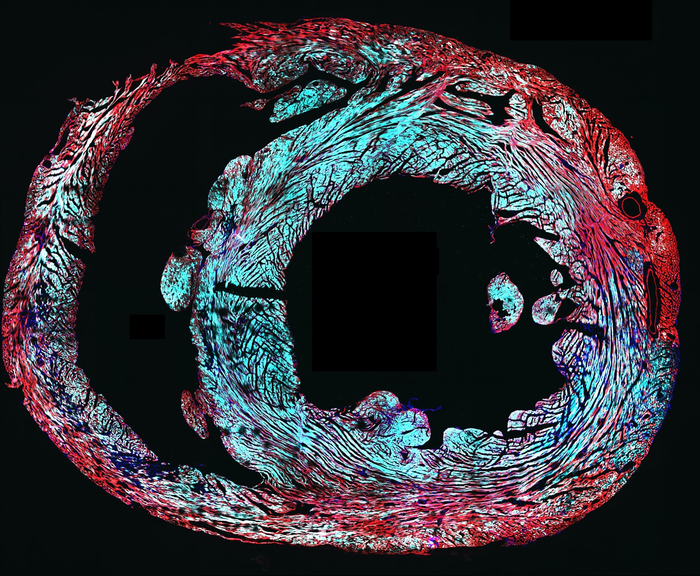
One possible treatment option for cardiac arrhythmias are approaches that enhance electrical excitability and action potential conduction in the heart. One way this could be done is by stably overexpressing mammalian voltage-gated sodium channels. However, the channels’ large size precludes delivery via viral vectors.

The work is published in Nature Communications, in the paper, “Engineered bacterial voltage-gated sodium channel platform for cardiac gene therapy.”
“We were able to improve how well heart muscle cells can initiate and spread electrical activity, which is hard to accomplish with drugs or other tools,” said Nenad Bursac, PhD, professor of biomedical engineering at Duke University. “The method we used to deliver genes in heart muscle cells of mice has been previously shown to persist for a long time, which means it could effectively help hearts that struggle to beat as regularly as they should.”
The platform, the authors wrote, “utilizes small-size, codon-optimized engineered prokaryotic sodium channels (BacNav) driven by muscle-specific promoters that significantly enhance excitability and conduction in rat and human cardiomyocytes in vitro and adult cardiac tissues from multiple species in silico.”
Several years ago, members of the lab mutated bacterial genes so that the channels they encode could become active in human cells. In this new work, Tianyu Wu, doctoral student, further optimized the content of the genes and combined them with a promoter that exclusively restricts channel production to heart muscle cells.
“We worked to find where the sodium ion channels were actually formed, and, as we hoped, we found that they only went into the working muscle cells of the heart within the atria and ventricles,” Wu said. “We also found that they did not end up in the heart cells that originate the heartbeat, which we also wanted to avoid.”
As a proof of concept, tests on cells suggested that the treatment improves electrical excitability enough to prevent human abnormalities like arrhythmias. More specifically, the work showed that “the expression of BacNav significantly reduces occurrence of conduction block and reentrant arrhythmias in fibrotic cardiac cultures.”

Within live mice, the results demonstrated that the sodium ion channels were active in the heart, showing trends toward improved excitability. Functional BacNav channels, the authors wrote, “are stably expressed in healthy mouse hearts six weeks following intravenous injection of self-complementary adeno-associated virus (scAAV) without causing any adverse effects on cardiac electrophysiology.” However, further tests are needed to measure how much of an improvement is made on the whole-heart level, and whether it is enough to rescue electrical function in damaged or diseased heart tissue to be used as a viable treatment.
As for next steps, the researchers have identified different bacterial sodium channel genes that work better in preliminary benchtop studies. The team is working with collaborators to test the ability of these genes to restore heart functionality in mouse models that mimic human heart diseases.
The large diversity of prokaryotic sodium channels, and experimental-computational platform reported in this study, “should facilitate the development and evaluation of BacNav-based gene therapies for cardiac conduction disorders.”
“I think this work is really exciting,” Bursac said. “We have been harnessing what nature made billions of years ago to help humans with modern-day disease.”