The global gene therapy market is growing rapidly. According to Polaris Market Research, the market, valued at $1.46 billion in 2020, is estimated to reach $5.02 billion by 2028. This growth is driven by the potential that gene therapies hold to address the rising prevalence of oncological and neurological disorders, as well as of rare genetic disorders. According to the National Center for Advancing Translational Sciences, gene therapy is particularly relevant to rare disease patients, as more than 80% of rare diseases have a known monogenic cause.
Gene therapy can permanently treat genetic diseases by replacing a missing or mutated gene with a functional copy. To do so, however, gene therapy requires a suitable delivery technology. Among the most promising delivery technologies is the adeno-associated virus (AAV) vector. An example of an AAV vector–based gene therapy is Novartis’ Luxturna®, the first gene therapy approved in the United States.
Since Luxturna® was approved in 2017 for the treatment of an inherited form vision loss, gene therapies have come to be developed at a quicker pace. Five first-time approvals are expected this year in the European Union alone. Also, several hundred gene therapies are in development.
To help ensure the success of new gene therapies, developers and manufacturers are working to consistently deliver high-quality products. Companies are offering new services, or adapting existing ones, to improve genetic characterization and quality control. Here, GEN speaks to several companies about the latest trends.
Meeting regulatory requirements
As new gene therapies have entered the marketplace, regulators have “raised the bar” on the research required to gain approval, according to Jennifer S. Chadwick, PhD, vice president of biologic development at BioAnalytix. “As the first AAV vectors came through the regulatory process, a lot of questions arose,” she recalls. “The expectation to address those questions with data is what we’re seeing now.”
Gene therapies are expensive. Luxturna, for example, has a list price of $425,000 per eye. With regulatory demands increasing, these high costs make analytics essential. “You need to show you’re making the right product, that it persists in the body, and that efficacy is long term,” Chadwick asserts. “Otherwise, you’re unlikely to get paid.”
Although reimbursement would be smoothed by analytics, the market for them remains underdeveloped. “The trend we see as an analytical solutions provider is there’s a real gap in analytics in the gene therapy space,” observes Susan Darling, senior director of CE and biopharma at Sciex. “Gene therapies are where monoclonal antibodies were years ago. They need better, faster, and stronger analytical solutions to characterize the product into manufacturing and quality control.”
A similar point is made by Behnam Baghbaderani, PhD, global head of process development, Cell and Gene Technologies, Lonza. “Some of the technologies used in current analytical methods are in the early development phase and face GMP compliance or regulatory challenges,” he maintains. “The pace of technological development may not be in line with the pace of growth.”
New capabilities for capsids
A critical factor in the effectiveness of gene therapy is whether AAV capsids contain the intended gene of interest. Therapeutic benefits of empty or partially filled capsids can be questionable, and/or they may have detrimental impact from safety and efficacy perspectives. According to Baghbaderani, this unwanted impact may be attributed to undesirable immune responses or competition with full capsids for vector binding sites on cells.
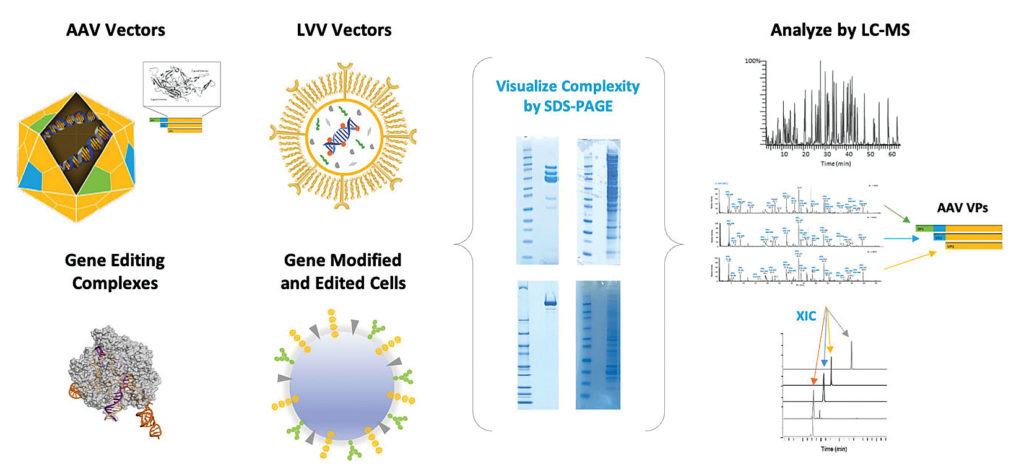
The main methods for detecting full and empty capsids are analytical ultracentrifugation (AUC) and liquid chromatography (LC). Analytical ultracentrifugation, Chadwick points out, is the “gold standard” method in this application area. It uses a specialized ultracentrifuge with optical sensors to monitor the size and density of capsids in solution.
“Although AUC gives you a simple readout, it can be quite a complex assay to apply,” Chadwick adds. “So, we also do a lot of LC separations.” Liquid chromatography–mass spectrometry (LC-MS) uses an LC instrument to separate sample components, which are fed into an MS instrument that can detect the mass, structure, and quantity of capsid proteins. LC-MS is becoming more popular for gene therapy analytics, she explains, because of the high level of detail it provides to AAV developers about the viral capsids and the specific attributes of the capsid proteins. Such information can suggest how well the capsids will perform in the body.
Results obtained with AUC or LC may be confirmed by transmission electron microscopy (TEM), a technique for viewing capsids directly. It works like light microscopy but uses a beam of electrons to create an image. An elaboration of TEM is cryogenic TEM (cryo-TEM), where samples are examined at cryogenic temperatures. It is gaining popularity in structural biology because it avoids the need for crystallization of macromolecules.
High-resolution cryo-TEM has been added to the services that Charles River Laboratories offers to gene therapy developers and manufacturers. “Cryo-TEM complements our negative-staining TEM capabilities,” states Rob Stachlewitz, PhD, corporate vice president, science and strategy lead, Global Lab Sciences, Charles River.
“Cryo-TEM,” he asserts, “will allow us to provide evaluation of capsid integrity and quantitation of empty/partial/full capsids without fixative and with a higher resolution than any other provider.” He adds that the number of empty/full/partial capsids may be measured if cryo-TEM is combined with artificial intelligence–based image analysis.
Assessing viral vector integration
Real-time or quantitative polymerase chain reaction (qPCR) technology is well established as a means of measuring viral titers. However, multiple studies have shown that droplet digital PCR (ddPCR) technology can generate more reproducible and statistically significant results. ddPCR uses a water–oil emulsion to divide nucleic acids among tens of thousands of droplets, each of which acts as a tiny test tube. Droplets may be arrayed on multiwell plates.
Although BioAnalytix focuses on protein content, Chadwick highlights ddPCR as a current trend in gene therapy analytics. “That’s what people are expecting now,” she says. “It’s so much more accurate.”
According to Bio-Rad Laboratories, ddPCR uses smaller sample sizes than qPCR because the PCR amplification is carried out on each droplet. Moreover, the subdivision of the sample occurs in a single step, due to the chemistry of the surfactants. This avoids not only the pipetting errors in serial dilution, but also the need for complex microfluidics.
“Recently, we implemented patent-pending ddPCR methods to our analytical capabilities as they can provide highly reliable data … for some of the key assays used to evaluate titers and to determine the identity and integrity of viral vector products,” Baghbaderani tells GEN. He notes that Lonza is moving its qPCR-based analyses of host cell contaminants to ddPCR.
Another company deploying ddPCR is Charles River, which is bringing the technology to all its laboratory sites during 2021. The company intends to take advantage of ddPCR’s capabilities in molecular biology and states that compared with traditional PCR, ddPCR provides “increased sensitivity and lower interference.”
Innovations in sequencing
Gene therapy development may benefit from applications of next-generation sequencing (NGS). For example, NGS may be used to confirm on-target effects or detect off-target effects, or to check whether a viral sequence has been correctly integrated into a cell line. NGS capabilities are being added to many companies’ service offerings. For example, Charles River is introducing NGS capabilities to its North American sites.
Cergentis offers a proprietary genetic sequencing technology called Targeted Locus Amplification (TLA). The company claims that TLA can sequence any transgene or locus of interest and allow for the detection of integration site(s) and of sequence and structural variants in the integrated transgene as well as in the surrounding host genome.
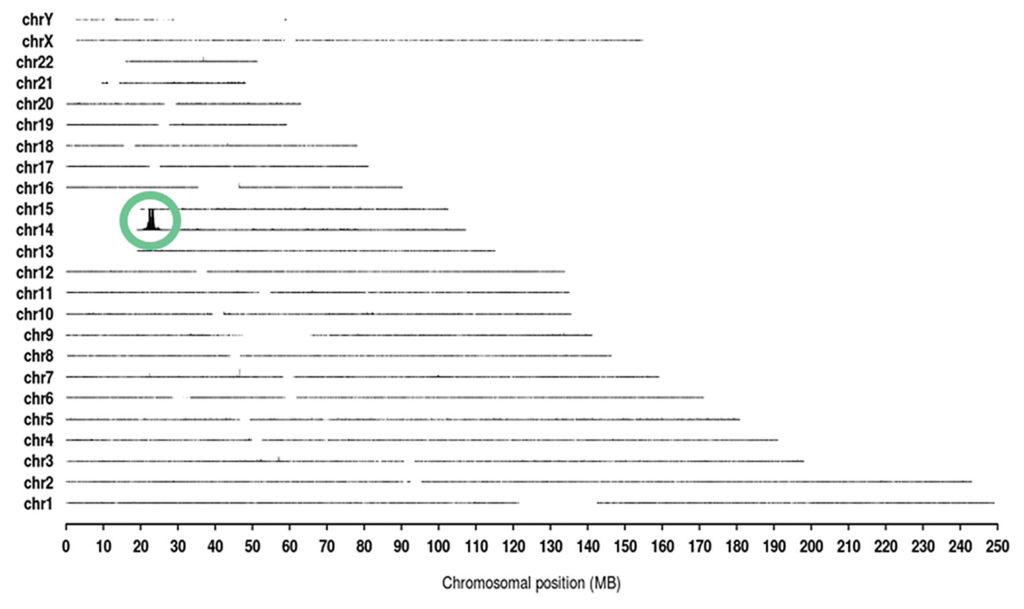
According to Judith Bergboer, PhD, head of services at Cergentis, TLA can help with quality control in upstream manufacturing by allowing companies to check whether a viral vector has correctly integrated into a cell line. TLA can also allow companies to check the genetic makeup of the virus at an early stage in cell line development—ensuring that there are no undesirable structural variants. Moreover, she says, the technology can be used to evaluate heterogeneous cell samples for the integration patterns and integrity of the transgene.
“The hallmark of our TLA technology is that it allows you to check both the [transgene] integration site and the integrity of the integrated sequence,” she emphasizes. “What is quite elegant is that you can see everything that’s interesting about a genetic locus. And with TLA, unlike a lot of technologies, you only need to know very limited sequence information on your locus of interest. TLA is hypothesis neutral.”
Embracing integration

“People don’t want a system that can only do one thing,” says Darling. “They want to grow, stretch, and move with changing pipelines.” A key benefit of the Sciex PA 800 Plus Pharmaceutical Analysis System, she asserts, is that it can do three gene therapy assays at once and also run assays for other therapeutics. “You can buy one platform to assess the transgene integrity and purity of AAV,” she continues, “and if you’re also developing bispecifics [bispecific monoclonal antibodies], you can analyze them on the same platform.”
A similar point is made by Baghbaderani. He notes that gene therapy developers are keen to streamline the move from small-scale development to commercialization: “It’s important to focus on incorporating automated, high-throughput analytical methods into the manufacturing process to allow more efficient and expedited development activities.”
He adds that gene therapy developers can benefit from working with a contract development and manufacturing organization such as Lonza. According to Baghbaderani, Lonza can offer extensive analytical expertise—ranging from early-stage development to downstream manufacturing—under a single roof.
Looking toward the future
Looking to the future, Baghbaderani sees gene therapy analytics as crucial to the development of streamlined, robust GMP-compliant manufacturing processes. Using process control analytics to ensure a consistency of product is also important, adds Chadwick.
Companies are also looking to provide a wider range of services. Sciex, for example, is repurposing its work on AAV vectors to meet a growing demand for assays designed for lentiviruses, Darling points out. The company is also looking into gene therapies that use liposomes instead of viral vectors, and it is developing techniques for RNA analysis. Cergentis is also developing a broader range of services. The company, Bergboer notes, is making its TLA analysis service available as a kit for in-house use.


