CRISPR excites ambition. Just consider a few CRISPR applications: gene drives (showing promise as a way to prevent malaria); ex vivo and in vivo gene therapies (entering clinical trials); and diagnostic tests (gaining momentum now that two assays have secured emergency use authorization for a raging pandemic disease—namely, COVID-19).
All of these applications can be traced back to research that was led by Emmanuelle Charpentier, PhD, and Jennifer Doudna, PhD. This research was introduced in a 2012 Science article that described the repurposing of a natural CRISPR system, an RNA-guided DNA endonuclease, as gene editing tool. Readers of the article may have noticed that its conclusion—that CRISPR “could offer considerable potential for gene targeting and genome editing applications”—was deliberately understated. Essentially, readers were invited to imagine how profound CRISPR applications might become, or how quickly these applications might leave the laboratory and enter the wider world.
Because CRISPR applications promise so many benefits, we are impatient to see them realized. Indeed, we may complain that the development of CRISPR therapies is too slow. Nonetheless, a handful of CRISPR therapies have advanced to the early stages of clinical trials, including therapies for sickle-cell anemia, HIV disease, and acute myeloid leukemia. And last year, a gene edited allogeneic chimeric antigen receptor T-cell therapy secured the FDA’s regenerative medicine advanced therapy (RMAT) designation for the treatment of relapsed or refractory CD19-positive B-cell malignancies.
We are eager to see CRISPR succeed not just in medicine, but in other application areas where humanity faces serious challenges—areas that include crop production, bioenergy, manufacturing, and environmental remediation. To hasten progress in all these areas, scientists are working diligently to add tools to the CRISPR toolbox. A selection of the most interesting new tools are presented in this CRISPR anniversary article.
Programmable gene insertion
Programmable and multiplexed genome integration of large, diverse DNA cargo independent of DNA repair remains an unsolved challenge of genome editing. Current gene integration approaches require double-strand breaks that stimulate DNA damage responses. Furthermore, CRISPR-based methods that bypass double-strand breaks, such as prime editing, are limited to modification or insertion of short sequences.
In late 2021, Jonathan S. Gootenberg, PhD, and Omar O. Abudayyeh, PhD, both alumni of the MIT laboratory led by Feng Zhang, PhD, presented their latest CRISPR system, PASTE (Programmable Addition via Site-specific Targeting Elements). This technology achieves efficient and versatile gene integration at diverse loci by directing insertion with a CRISPR-Cas9 nickase fused to both a reverse transcriptase and a serine integrase. Without generating double-strand breaks, PASTE can integrate sequences as large as ~36 kb with rates of between 10 and 50% at multiple genomic loci in human cell lines, primary cells, and quiescent nondividing primary human cells.

“The PASTE technology enables programmable gene insertion and the ability to put large cargos into the genome anywhere you want,” Gootenberg said. “It’s the final frontier for therapeutics but also cell engineering and basic biology because we can craft the genome any way we want. I have this enormous piece of cargo, and I can drop it in [the genome] where I want.”
One of the best applications of programmable gene insertion is in therapeutics. “There are companies that are using advanced CRISPR approaches that make single-nucleotide or small edits,” Gootenberg remarked. These approaches are fascinating, he noted, but he added that they may not work with genes that harbor many mutations.
“The cystic fibrosis transmembrane conductance regulator gene, for example, has about 1,800 different mutations,” Gootenberg pointed out. “You’re not going to develop 1,800 different drugs for cystic fibrosis. So, instead of taking that approach, you could replace the gene entirely with a healthy one. Then you don’t even need to know what mutation the patient has.”
Cell-type-specific delivery

Arguably the biggest challenge in realizing the full potential of CRISPR, according to not only Gootenberg and Abudayyeh but also Doudna, is improved in vivo delivery. She believes we’ll see it in the next decade. Doudna told GEN, “To reach the point where CRISPR is the standard of care for all the types of diseases we know it can address, we need to be able to target more cell and tissue types precisely.” Doudna said that this is important for developing therapies that are affordable and accessible.
For diseases that require systemic administration of CRISPR-Cas9 systems, cell-type-specific delivery is ideal to avoid delivery-mediated off-target effects. Although viral and nanoparticle delivery systems have shown promise, especially for delivery to the liver, there’s a lot to be done in other cells or tissues, especially to enhance selectivity. Efforts to achieve cell-type-specific gene delivery with nonviral vectors have spanned surface modification by various targeting moieties, such as small-molecule ligands, peptides, and antibodies. One route is to use aptamers, single-stranded oligonucleotides that fold into defined architectures and bind to targets such as proteins.
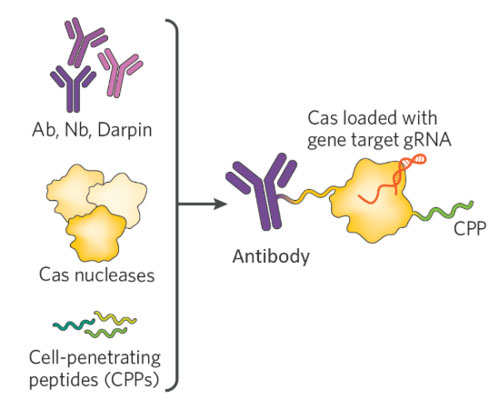
Spotlight Therapeutics is addressing multiple limitations of the current in vivo delivery systems by developing engineered ribonucleoproteins with its Targeted Active Gene Editing (TAGE) platform. These ribonucleoproteins are created by combining transmembrane trafficking moieties (cell-penetrating peptides) and a cell-targeting moiety (ligand or antibody) with a CRISPR nuclease.

Spotlight
“We took a biologics approach, and our architecture is riffing off the success of antibody conjugates for dragging payloads to selected cells,” said Mary Haak-Frendscho, PhD, president and CEO of Spotlight. “We are using antibodies or antibody derivatives to deliver a nuclease effector (Cas9 or Cas12) to a selected cell type already loaded with a guide.”
These biologics have a short half-life and do not persist in vivo after the desired gene editing event. This reduces the risk of off-target cleavage events, minimizes the potential for antidrug immune responses, and allows greater flexibility in dosing.
Gene silencing/activation
The epigenome, nature’s gatekeeper to gene expression, consists of markers that are added or removed by very elegant and highly conserved mechanisms to determine the identity and function of each cell. For example, these markers do a great job of ensuring that our neurons stay neurons throughout our lifetimes. By targeted modulation of the epigenome, gene expression can be tweaked without ever cutting or breaking the DNA, which avoids some collateral damage that can come from using gene editing.

Chroma Medicine
According to Catherine Stehman-Breen, MD, CEO of epigenetic medicines developer Chroma Medicine, genetic regulation via gene editing is like stopping your car by crashing into a light post. “The horn is blaring, the windshield is broken, and the car is stopped,” Stehman-Breen explained. “You would have had a lot less damage if only you had used the brake—the device that’s meant to stop the car. That’s how I think about epigenetics.”
Chroma Medicine is providing a modular, flexible, CRISPR-based platform for developing epigenetic medicines based on the silencing or activation of genes.
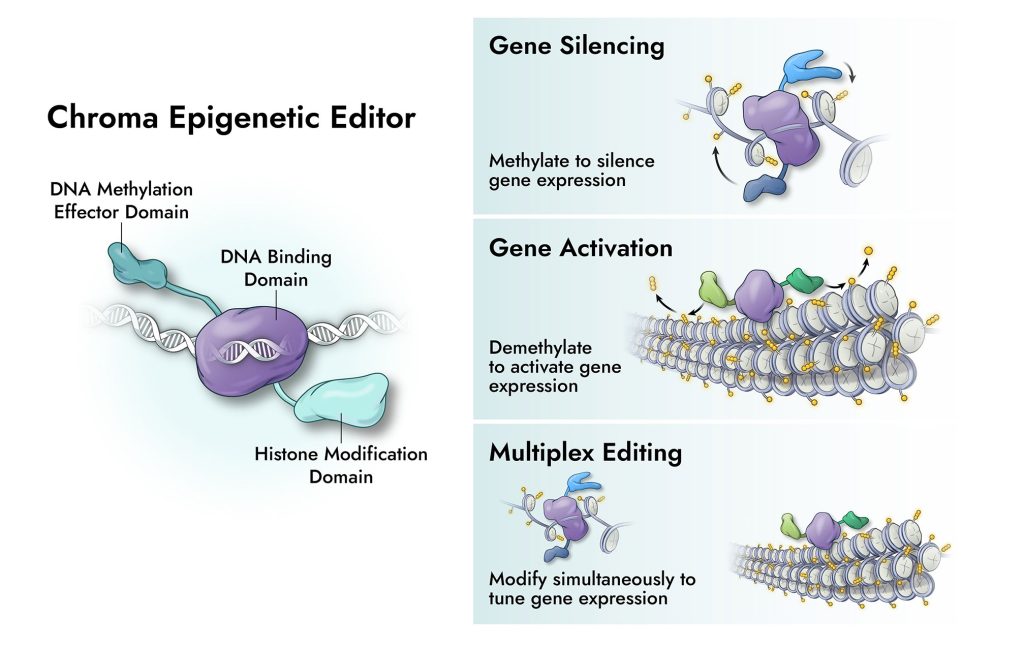
The platform couples a DNA binding domain with epigenetic effector domains. The DNA binding domain specifically targets the gene (or genes) to be silenced or activated. The effector domains create specific methylation patterns which control chromatin conformation and govern whether a gene is accessible or inaccessible for transcription. In simple applications, single genes may be targeted. In more complex applications, multiple genes may be targeted at the same time. It is even possible for some genes to be silenced while others are activated.
Stehman-Breen said that the complex multiplexing that Chroma’s platform offers can be used in a host of ways. For example, these tools can be used in cell therapy, where it may not be ideal to do complex multiplexing with gene editing since some of the challenges, such as transpositions, start to become exponentially more difficult. “With epigenetic modulation,” Stehman-Breen remarked, “you can manipulate as many genes as you want without resulting in any cuts or mixing of the DNA.”
Microbiome modulation
A growing trend in the CRISPR world relates to the microbiome. CRISPR is being packaged into phages to affect bacteria in this setting, which is the opposite of how the CRISPR system exists in nature—bacteria use CRISPR to fight off phages. Irony aside, CRISPR technologies have the potential to enable unprecedented control over microbiome populations. For example, one may eliminate drug-resistant bacteria without targeting beneficial bacteria. This kind of control is critical to addressing antibiotic resistance, which is spreading rapidly around the world and seriously impeding efforts to control microbial infections.
Killing selected bacterial populations just one way to exert control over the microbiome. Another way is to genetically modify bacteria so that they express therapeutic proteins and peptides. The second form of control raises the prospect of hijacking bacteria to modulate critical processes such as metabolic and immunological processes.

Snipr Biome
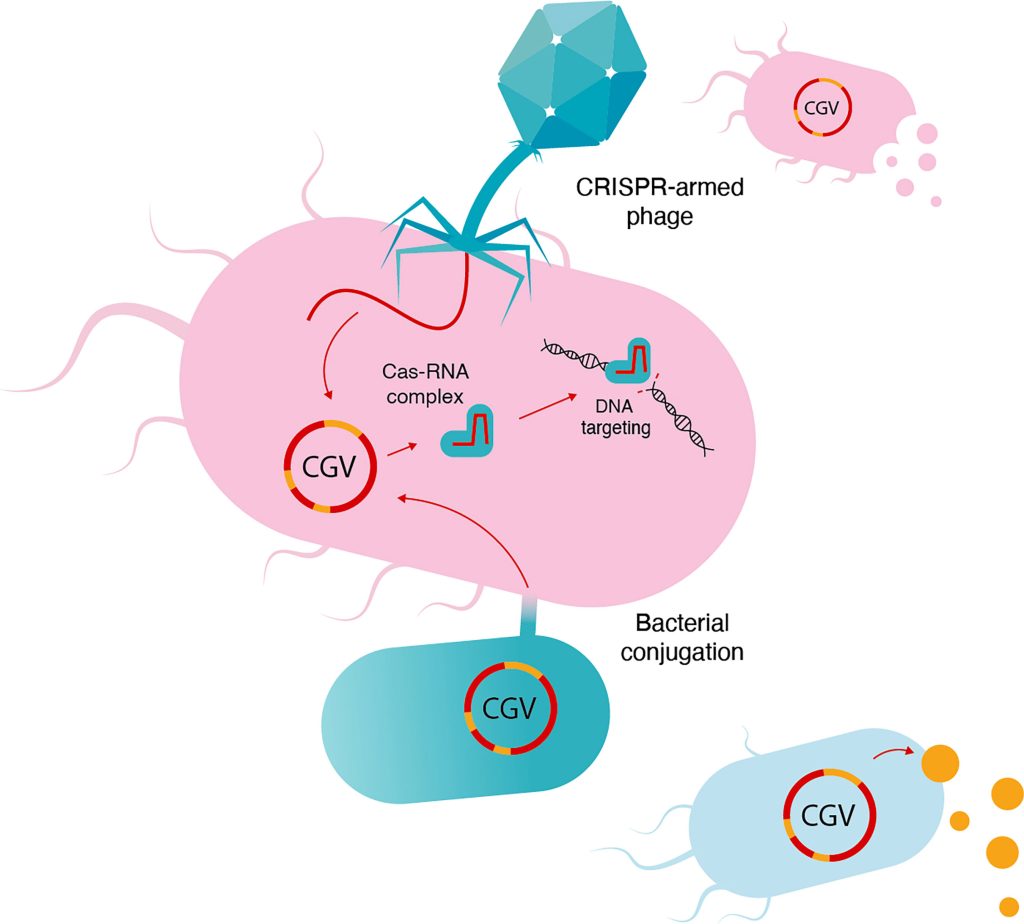
Both microbiome control approaches are being explored at Snipr Biome, a Danish company that has developed a proprietary technology called CRISPR-Guided Vectors. “We can modulate the microbiome by inserting genes that express proteins, antibodies, enzymes, or small hormones to achieve a therapeutic output,” said Christian Grøndahl, PhD, Snipr Biome’s co-founder and CEO. He added that the company’s technology could be used to modulate the microbiome and treat diseases such as type 2 diabetes, obesity, nonalcoholic fatty liver disease, and irritable bowel disorder.
Safety is a big concern when making a chronic and live treatment. “If you introduce a gene,” Grøndahl pointed out, “you also have to be able to control it and shut it off when you have obtained your treatment effect or when you want to lower the output.” To do so, Snipr Biome is using its technology to install “safety switches” that allow inserted genes to be excised—a better alternative to harvesting the microbiome completely.
“Although you want to go in and modulate the microbiome, you don’t want to disturb the pristine diversity of the microbiome,” Grøndahl stated. “Therefore, it’s not a good idea just to give antibiotics to cut off your treatment.”
The next 10 years of CRISPR
In the next 10 years, we will likely see a growing number of CRISPR-based diagnostics and approved medicines. We may even see people treated safely and effectively for genetic diseases we thought would never be curable. It’s possible that there will be so much headway made in the CRISPR space on literal and metaphorical bandages, that people will be able to preemptively alter the health of individuals.
Along these lines, Doudna’s take on the therapeutic use of CRISPR in the next decade isn’t for treatment—it’s for prevention. “There has been a lot of attention on CRISPR’s potential to treat specific diseases, but I also think that we’ll start seeing new applications to prevent disease in the coming years,” Doudna related. “We’re already seeing CRISPR being used as a diagnostic, and we’ve all seen how important fast, accurate diagnostics are to prevent the spread of disease. But we can also edit genes that predispose people to disorders, including diseases of aging.”
With all of the excitement in the healthcare space, it’s easy to overlook all of the potential good that can be done for the future of the planet. While there’s plenty to be excited about regarding the clinical applications of CRISPR, Doudna thinks that the agriculture and climate applications have the potential to have an even more significant impact worldwide.
“We’re starting to see CRISPR-edited agricultural products now,” Doudna noted. “We’ll see many more over the coming years addressing issues like food security, drought and flood tolerance, reducing pesticide and fertilizer use, eliminating agricultural emissions, as well as carbon removal and sequestration.”
For all of the uncertainty that lies ahead, one thing we can all be sure about is that CRISPR will likely have a role in shaping the future of individuals and the planet.


Comments are closed.