Gene drives have received their fair share of controversy in the past several years, as the technology has evolved to the point of where it could push a species to the point of extinction, which has been argued by some—in the case of malaria-carrying mosquitoes—to be an ecological risk worth taking. Yet, gene drives need not be an all-or-nothing approach with respect to pathogen resistance. The investigators at the Wyss Institute for Biologically Inspired Engineering, within Harvard University, have developed a CRISPR/Cas9-based gene drive platform to address growing drug resistance in the fungal pathogen Candida albicans. Findings from the new study were released today in Nature Microbiology, in an article entitled “A CRISPR–Cas9-Based Gene Drive Platform for Genetic Interaction Analysis in Candida albicans.”
C. albicans is a notorious human fungal pathogen that causes thrush and serious systemic infections. Opportunistic C. albicans fungi, which often live inconspicuously in the normal flora of human skin and gut, can switch from their harmless stealth mode to become aggressive pathogens, especially in people whose immune systems are already compromised by pre-existing diseases or harsh drug therapies. They can also form biofilms on medical devices, such as catheters and stents in the human body, leading to infections and sometimes death. The threat posed by both free and biofilm-bound forms of the pathogen is constantly growing, as virulent C. albicans strains are becoming increasingly resistant to the few drugs that are available to treat them.
Since the pathogenic yeast are diploid organisms—as they typically contain two copies of their entire genome—they are notoriously difficult to eliminate. To understand the role that a specific gene plays, researchers need to be able to delete both copies at the same time, allowing them to observe the effects of the gene's total absence, which has been a difficult challenge in C. albicans. Additionally, genes often play very similar and sometimes redundant roles in many processes, including drug resistance and biofilm formation, meaning that more than one gene needs to be deleted to identify those genes whose functions are linked.
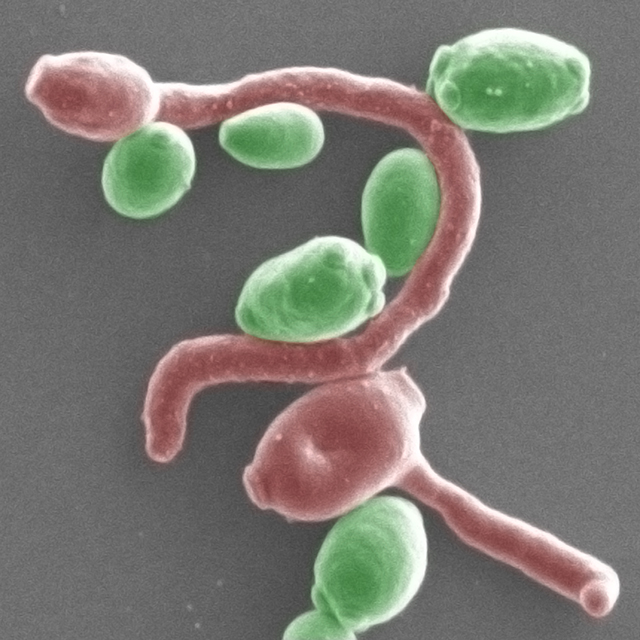
![This image shows multicolored diploid <i>Candida albicans</i> fungi growing on an agar plate with red-colored colonies indicating that two copies of a marker gene have been effectively deleted by the gene drive. [Wyss Institute at Harvard University]” /><br />
<span class=](https://genengnews.com/wp-content/uploads/2018/08/154498_web2309780176-1.jpg) This image shows multicolored diploid Candida albicans fungi growing on an agar plate with red-colored colonies indicating that two copies of a marker gene have been effectively deleted by the gene drive. [Wyss Institute at Harvard University]
This image shows multicolored diploid Candida albicans fungi growing on an agar plate with red-colored colonies indicating that two copies of a marker gene have been effectively deleted by the gene drive. [Wyss Institute at Harvard University]
Noted Wyss Institute professors George Church, Ph.D., and James Collins, Ph.D., along with their colleagues, approached the gene deletion challenge in C. albicans by creating a CRISPR gene drive platform for developing diploid strains of the pathogen in which both gene copies could be efficiently deleted. The researchers are hopeful that the technique may lead the way toward a better understanding of drug resistance and biofilm-forming mechanisms, and through future research, it could help pinpoint new drug targets and combination therapies.
The team took advantage of a recently discovered very rare haploid form of C. albicans, which, like those of other fungi, only contains one set of chromosomes with one copy of each gene, but they can be mated to easily create the diploid form
“We used haploid C. albicans strains and replaced genes that we wanted to eliminate with a gene drive that we previously developed and adjusted to the specific biology of C. albicans,” Dr. Church explained. “After mating, these 'selfish genetic elements' proceed to replace the normal copy of the gene in the diploid fungi. The approach worked so efficiently that it enabled us to even delete pairs of different genes simultaneously with higher throughput and to explore whether their functions are related.”
The new gene drive approach is based on the CRISPR/Cas9 system, in which a DNA-cutting Cas9 enzyme is targeted to two regions that flank a gene in haploid C. albicans fungi by two so-called guide RNAs (gRNAs). After the targeted gene sequence has been cut out, an engineered gene drive cassette expressing all Cas9 and gRNA components is inserted in its place. When two haploid fungi are mated to form diploid offspring, the gene drive will also substitute the gene's counterpart in the other chromosome, effectively deleting the original version from the organism entirely. By applying their gene deletion approach, the team was able to identify combinations of genes that act synergistically in defying certain drugs, or in triggering biofilm formation.
“For example, deleting either the two-efflux pump-encoding genes CDR1 and CDR2, or TPO3 and CDR2 together, rendered C. albicans highly sensitive to fluconazole and other antifungal drugs, suggesting that targeting two mechanisms at the same time could help overcome drug resistance,” noted co-lead study investigator Rebecca Shapiro, Ph.D., a postdoctoral fellow in Dr. Collins' team. “In biofilm formation assays, we also found that loss of the ALS3 adhesion factor gene synergizes with the loss of several other adhesion factor genes, which makes it a highly interconnected hub of biofilm adhesion and an interesting candidate to further explore.”
The investigators were excited by their findings and are extremely optimistic that their study will offer new inroads into understanding the difficult territory of C. albicans pathogenesis and drug resistance.
“We can now get a much better handle on how genetic networks that underlie the virulence of C. albicans are organized, see how they respond to specific environmental and drug perturbations, and thereby uncover new vulnerabilities that in the future may lead to new drug targets and combination therapies,” concluded Dr. Collins. “Moreover, our gene drive array platform can be a blueprint for similar approaches in other fungal pathogens, such as the newly emerging Candida auris, which is highly drug-resistant and has already been marked as a threat by the CDC.”