Researchers from the University of Sheffield say they have identified a new potential treatment pathway for cardiovascular disease. Their study (“Myeloid Tribbles 1 induces early atherosclerosis via enhanced foam cell expansion”), which appears in Science Advances, reportedly has shown for the first time that a protein expressed in a subset of immune cells contributes towards the build-up of fatty deposits in arteries, which leads to cardiovascular disease.
These fatty deposits are caused by macrophages, which are known to take up surplus cholesterol. When this is present in excess, they mature into larger cholesterol-laden cells known as foam cells which accumulate and cause blockages inside arteries.
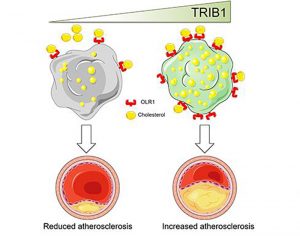
“Macrophages drive atherosclerotic plaque progression and rupture; hence, attenuating their atherosclerosis-inducing properties holds promise for reducing coronary heart disease (CHD). Recent studies in mouse models have demonstrated that Tribbles 1 (Trib1) regulates macrophage phenotype and shows that Trib1 deficiency increases plasma cholesterol and triglyceride levels, suggesting that reduced TRIB1 expression mediates the strong genetic association between the TRIB1 locus and increased CHD risk in man,” the investigators wrote.
“However, we report here that myeloid-specific Trib1 (mTrib1) deficiency reduces early atheroma formation and that mTrib1 transgene expression increases atherogenesis. Mechanistically, mTrib1 increased macrophage lipid accumulation and the expression of a critical receptor (OLR1), promoting oxidized low-density lipoprotein uptake and the formation of lipid-laden foam cells.
“As TRIB1 and OLR1 RNA levels were also strongly correlated in human macrophages, we suggest that a conserved, TRIB1-mediated mechanism drives foam cell formation in atherosclerotic plaque and that inhibiting mTRIB1 could be used therapeutically to reduce CHD.”
The findings of this early translational study which involved the University of Leicester and scientists from Hungary and the United States, suggest that inhibiting TRIB1 in macrophages could be a viable therapeutic target in treating cardiovascular disease.
Researchers have long been trying to identify the proteins regulated by TRIB1 to understand their effects, and whether they are of benefit or are detrimental to disease development.
Jessica Johnston, PhD, from the department of infection, immunity, and cardiovascular disease (IICD) at the University of Sheffield was first author of the study and her PhD research focused on the project.
“The role of TRIB1 in macrophages has remained elusive for some time,” she said. “Our research provides the missing link and highlights the importance of cell-specific expression in cardiovascular disease.”
According to Endre Kiss-Toth, also from the department of IICD, “Studying the genetics of cardiovascular disease in large human populations has revealed that TRIB1 contributes to its development.
“However, this is the first time that its role in immune cells has been directly addressed, thus uncovering a new mechanism by which arterial disease develops.
“The research into this mechanism has not yet translated into novel medical interventions. However, we now have preclinical proof that it would be beneficial to build on this research and see which patients with cardiovascular disease would benefit from the development of treatments to manage their lipid-laden foam cell formation.”