Cancer continues to claim a staggering number of lives. It is the leading cause of death worldwide and in the United States. Yet the statistics are not entirely grim. In the United States, the death rate peaked in 1991 and has steadily decreased since.
This encouraging trend may be strengthened now that immunotherapies are being deployed more widely. Immunotherapies are already revolutionizing the treatment of cancer—not just blood cancers, but solid cancers, too.
Despite the pandemic, immunotherapy continues to inspire hope and excitement, attitudes that serve to sustain the field and drive it forward. Hope and excitement are certainly evident among the scientists scheduled to speak at the 17th Annual PEGS Boston—The Essential Protein Engineering & Cell Therapy Summit. This event, which will be held virtually May 2021, will highlight the newest approaches in the field.
Next-generation targets and strategies include investigating the power of a patient’s own tumor-infiltrating lymphocytes (TILs); developing an allogeneic T-cell therapy using a dimeric antigen receptor to target an overexpressed tumor protein; enhancing innate immunity to remodulate the tumor microenvironment; and generating an engineered interleukin-15 (IL-15) for expanding killer cell responses.
“Stealth” lymphocyte attack
In the early stages of a tumor’s development, host TILs attack the foreign mass by penetrating its stroma. This natural defense does not always work. “If there are genetic or environmental factors affecting tumor growth, the tumor cells may prevail,” explains Maria Fardis, PhD, president and CEO of Iovance Biotherapeutics. “Cancer cells may also suppress the natural antitumor response of the body by expressing cell surface proteins such as PD-L1 or CTLA-4.”
Iovance’s platform utilizes a patient’s own TILs as a stealth-like technology for immune defense against solid tumors. “Instead of targeting one preselected antigen, as with many other types of therapy, we isolate and expand a patient’s TILs, which target a diverse array of cancer antigens,” Fardis elaborates. “We infuse the TILS back into the patient to attack the cancer.”
The process first entails removing a section of the tumor (~1.5 cm) with surgical resection. TILs from the sample are expanded to billions of cells via Iovance’s streamlined proprietary 22-day process in the company’s GMP-compliant manufacturing facility. Fardis asserts, “Removing cells from the immunosuppressive tumor environment and growing the cells ex vivo activates and expands the patient’s polyclonal TILs.”
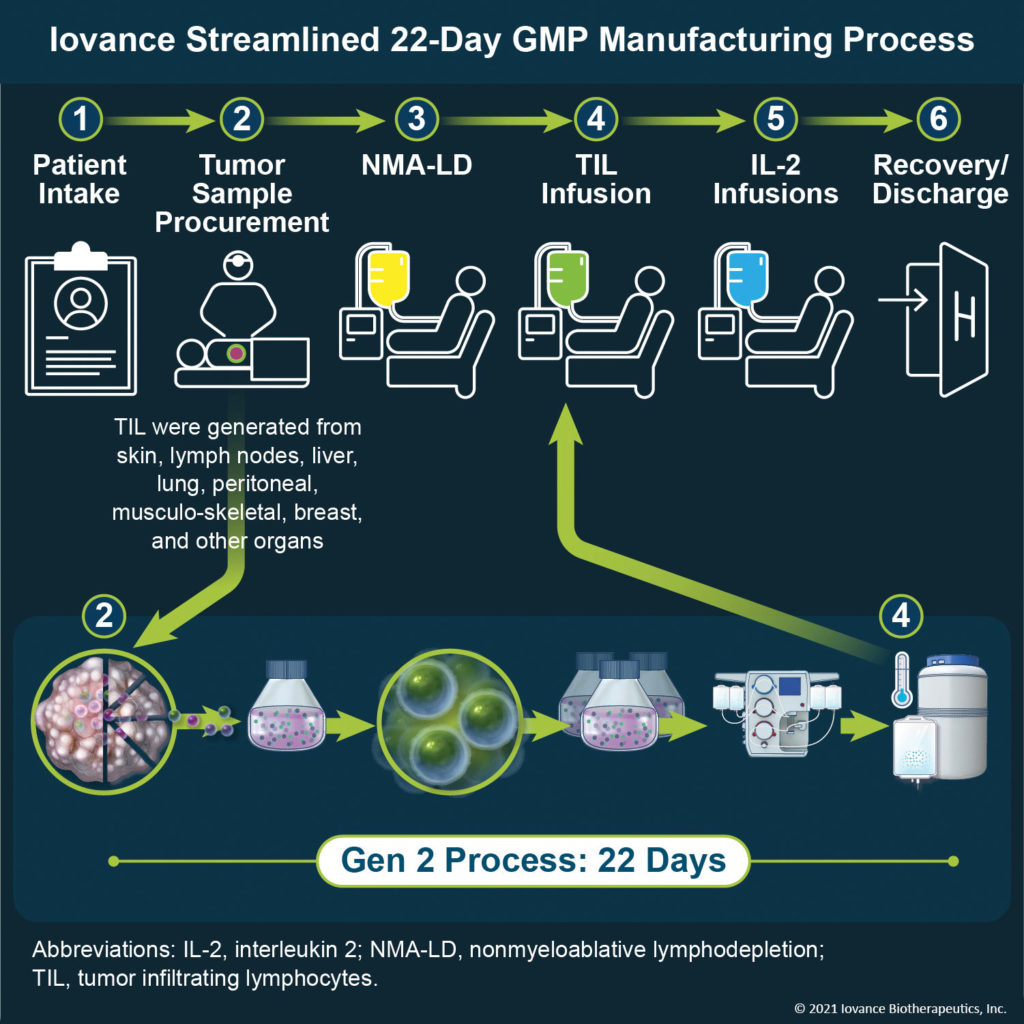
Prior to TIL infusion, the patient is preconditioned to remove suppressive factors. “We have achieved a 90% success rate in TIL manufacturing and have dosed greater than 400 patients,” Fardis notes. “What’s remarkable is that the TIL approach enables a one-dose treatment and requires no follow-up treatment. In our melanoma clinical trial, for example, after a single infusion of TILs, 36% of late-stage patients demonstrated a response. Even after 28 months, these patients have not yet reached the median duration of response. This could mean the TILs are still active and working!”
In addition to melanoma, Iovance is pursuing a number of ongoing Phase II trials including those for cervical cancer, head and neck cancer, and non-small cell lung cancer.
The company continues to refine and expand its TIL technology platform, and it hopes to see TILs become part of cancer adjuvant therapy in the future. “To prevent metastasis of a newly diagnosed cancer, TILs could be given after tumor removal as part of treatment,” Fardis explains. “Another potential application is to genetically modify TILs to remove the ‘brakes’ on the natural immune response, such as the well-known PD-1 and CTLA-4 pathways. TIL therapy could represent the utmost in personalized medicine as well as a revolutionary, accessible, and mainline cancer therapy.”
“DAR T-ing” for a cure
Autologous chimeric antigen receptor (CAR) T-cell therapy has shown great promise, especially for the treatment of hematological malignancies. However, several CAR T-cell therapy challenges continue to vex the field. For example, therapies typically rely on patient cells, and manufacturing remains difficult.
To help overcome these challenges, Sorrento Therapeutics has developed an allogeneic T-cell therapy based on genetic engineering of donor-derived T cells to express a dimeric antigen receptor (DAR). One of the company’s DAR T-cell programs focuses on targeting GD2, a disialoganglioside that has limited expression in normal tissues but is overexpressed across a wide range of tumors.
“GD2 is a well-characterized tumor antigen in neuroblastoma, which is also expressed on osteosarcomas and some other sarcomas,” comments Wenzhong Guo, PhD, CTO, Cell Therapy, Sorrento. “The prognosis for advanced neuroblastoma remains poor with a high risk of recurrence even after multiple therapies. Therapies based on monoclonal antibodies that specifically target GD2 on tumor cells are improving treatment results for high-risk neuroblastoma.”
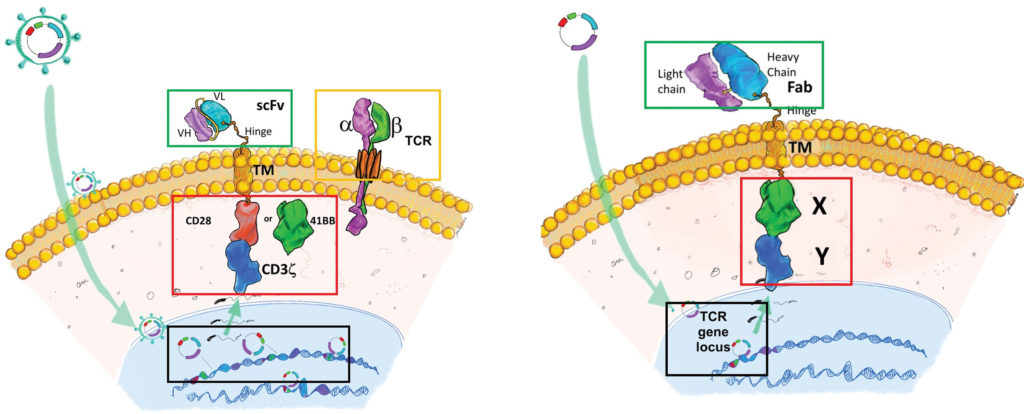
Sorrento is developing second-generation anti-GD2 allogeneic DAR T cells to target GD2 for improving the efficacy of CAR T-cell therapy. Guo explains, “In the DAR structure, a Fab antibody is used to replace the scFv antibody of the CAR structure. Preclinical studies show this modification can improve specificity and antitumor activity.
“Further, a proprietary one-step ‘knock out/knock in’ (KOKI) approach is used to integrate the DAR construct into the T-cell receptor locus, which eliminates viral vector transduction, saving product development time and reducing manufacturing costs. This approach can be used to produce both autologous and allogeneic DAR T-cell products.”
Guo believes the approaches are not mutually exclusive: “Although allogeneic CAR T cells can resolve some current challenges of autologous CAR T cells, allogeneic approaches are associated with two major issues. First, the administered allogeneic T cells may cause life-threatening graft-versus-host disease. Second, these allogeneic T cells may be rapidly eliminated by the host immune system, limiting their antitumor activity.
“Multiple approaches have been used to resolve these issues; however, the persistence of autologous CAR T cells is typically better than that of allogeneic CAR T cells. Allogeneic and autologous CAR T-cell therapies may each have therapeutic benefits, depending on the desirability of CAR T-cell persistence for a given indication.”
Sorrento’s preclinical studies are targeting neuroblastoma, osteosarcoma, and other cancers. The company have filed an IND application for its allogeneic anti-CD38 DAR T-cell program.
Enhancing immunotherapy response
Although immune checkpoint inhibitors such as CTLA-4 and PD-1 blockers can achieve significant survival benefits against metastatic melanoma, poorly immunogenic tumors require additional modalities such as strong co-stimulatory signals to amplify T-cell signaling for maximal antitumor response.
A number of strategies are being employed to manipulate co-stimulatory signals to enhance T cell–mediated tumor attack. For example, activation of NKG2D, an activating immune surveillance receptor, assists in the elimination of abnormal cells. NKG2D is expressed primarily on the cytotoxic arm of the immune system: natural killer (NK) cells, CD8+ T cells, natural killer T cells (NKTs), and subsets of γδ T cells.
In humans, NKG2D is activated via binding to the family of ligands for the major histocompatibility complex (MHC) class I chain–related molecules referred to as MICs. Usually, MICs are expressed only on cells under oncogenic or environmental challenge, and not on healthy cells. Further, a tumor-derived soluble version of an MIC (sMIC) serves as a decoy ligand that impairs NKG2D-mediatedNK cell tumor-killing ability and co-stimulation of tumor-killing CD8+ T cells.
Jennifer Wu, PhD, professor of urology and immunology, Feinberg School of Medicine, Northwestern University, investigated the NKG2D-MIC axis in association the immunosuppressive effects of sMIC during PD-1/PD-L1 blockade and CTLA-4 blockade therapy. She and colleagues tested the hypothesis that antibody targeting of sMIC could enhance the therapeutic efficacy of PD-1/PD-L1 blockade and CTLA-4 blockade.
They assessed a single-agent therapy of a PD-1/PD-L1 or CTLA-4 blockade antibody or a nonblocking antibody that targeted sMIC, or a combination therapy of PD-1/PD-L1 or CTLA-4 blockade antibody and the sMIC-targeting antibody. The therapies were administered to well-characterized preclinical MIC/sMIC+ tumor models that closely resembled the NKG2D-medated oncoimmune dynamics of MIC+ cancer patients.
The team found that antibody co-targeting of sMIC enhanced responses of sMIC+ tumors to the PD-1/PD-L1 blockade and the CTLA-4 blockade. Further, using combination therapy enhanced antigen-specific CD8+ T-cell enrichment and antitumor function in tumors. Wu and colleagues proposed that the study provides proof of concept that targeting sMIC with a nonblocking monoclonal antibody can enhance responses to PD-1/PD-L1 therapy or CTLA-4 therapy on sMIC+ tumors.
IL-15 agonist
The primary curative option for patients with advanced hematological malignancies is allogeneic hematopoietic cell transplantation. Despite this treatment, patients often have a poor prognosis and relapse rates of nearly 50%. Nektar Therapeutics is pursuing the strategy of enhancing antitumor immunity by utilizing an engineered version of IL-15 to stimulate natural immunity.

Nektar Therapeutics
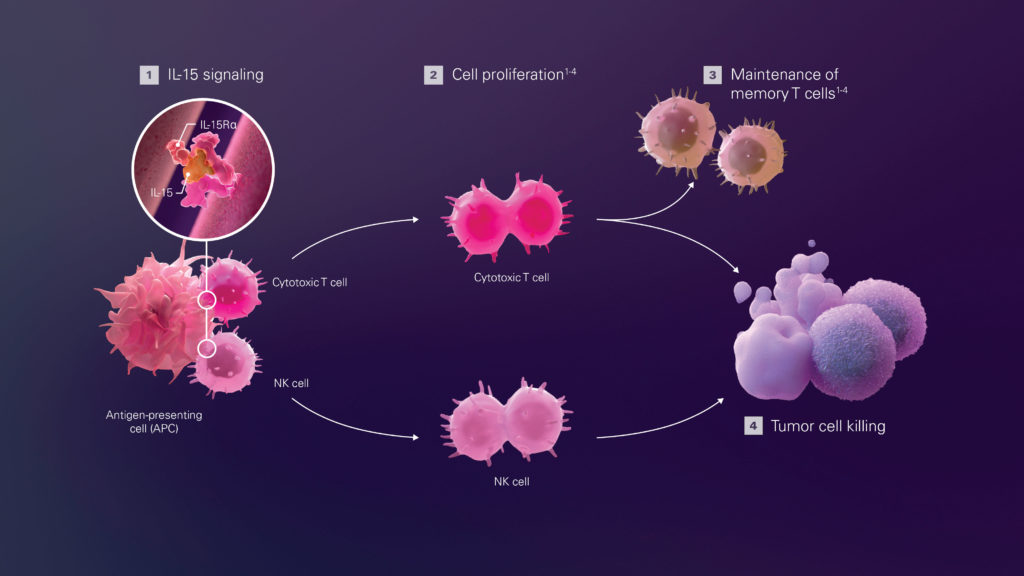
“Activation of the IL-15 pathway can promote antitumor responses as it enables the expansion, survival, and function of NK cells and memory CD8+ T cells without inducing suppressive regulatory T cells,” says Willem Overwijk, PhD, vice president of oncology research at Nektar. “However, native IL-15 has some important drawbacks that have limited its application in cancer therapy. For example, it has a very short half-life in the circulation, necessitating frequent administration of high doses that can lead to toxic side effects as well as desensitization over time.”
The company’s drug candidate under development, NKTR-255, is an engineered version of native IL-15. It employs advanced polymer chemistry and is designed to capture the full IL-15 pathway activity and increase cytotoxic function against cancer cells, while also providing a highly improved pharmacokinetic profile. According to Overwijk, the polymer chemistry “lets NKTR-255 remain active in the bloodstream longer, improving tolerability and allowing for administration once every 2 to 4 weeks.”
Overwijk notes that NKTR-255 is advantageous in two respects: “By targeting the natural IL-15 pathway, NKTR-255 increases the number and activity of NK cells and CD8+ T cells, which mediate direct antitumor activity in preclinical models of cancer. In addition, NK cells activated by NKTR-255 can efficiently recognize specific antibody therapeutics that bind to cancer cells, which could further increase their efficacy and thus lead to better patient outcomes.”
NKTR-255 is currently being evaluated in a Phase I study in adults with relapsed/refractory non-Hodgkin lymphoma or relapsed/refractory multiple myeloma (NCT04136756) and in a Phase Ib/II study in combination with cetuximab as a salvage regimen for solid tumors (NCT04616196).
Nektar has a number of investigational medicines in different stages of clinical development. Overwijk reports, “Nektar continues to build upon its deep understanding of immunology to discover and develop novel immunomodulatory therapies for the treatment of cancer, autoimmune disorders, and chronic inflammatory conditions.”
