There is perhaps no better example of getting to the root of a problem than gene therapy. This medical treatment alters the very building blocks of life, and it has the potential to treat or cure not just genetic diseases, but cancers and infectious diseases as well.
For years, scientists have been focusing on whether or not these treatments can work. Now armed with evidence in the affirmative, they have been devoting more of their attention to exploring the safest and most efficient ways of introducing these treatments to host cells.
For these types of treatments, viruses seem like the obvious choice, as they are designed to infiltrate cells. As such, all FDA-approved gene therapies are delivered using viral vectors, including viral vectors based on adeno-associated virus (AAV), lentivirus, or herpes simplex virus. However, while viral vectors have met with some success, they have also raised doubts. Potential risks of viral vectors include liver toxicity, immunogenicity, and oncogenicity. In addition, viral vectors have a relatively small packaging capacity, and they’re difficult to produce at scale because they rely on costly and time-
consuming manufacturing processes.
To skirt the limitations associated with viral vectors, scientists are turning to nonviral delivery systems. However, unlike viruses, nonviral delivery systems lack the natural ability to enter cells. Instead, nonviral delivery systems must rely on physical or chemical mechanisms.
Transforming the eye into a biofactory
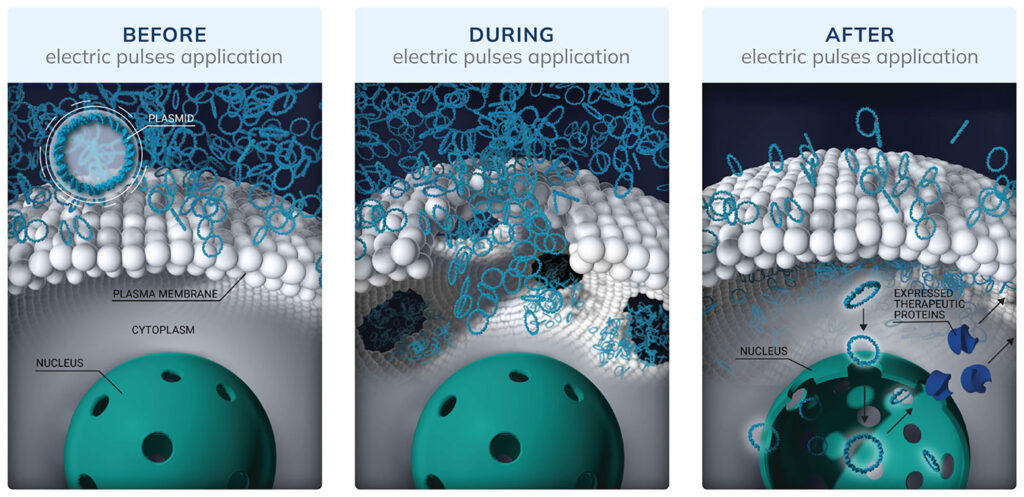
Because the eye is accessible and immune privileged, it is a prime target for physical delivery approaches such as the transfer of genetic material via electroporation, which uses an electrical pulse to create temporary pores in cell membranes. With electroporation, genetic material such as DNA plasmids can overcome natural barriers of the eye that often thwart viral vectors. Also, DNA plasmids can target specific types of retinal cells.
“Viral vectors, when delivered into the vitreous, may not effectively target specific retinal cell types or regions, leading to nonspecific gene expression,” observes Thierry Bordet, PhD, chief scientific officer at Eyevensys. “This lack of cell-type specificity can result in off-target effects or inadequate transduction of the desired retinal cells, thereby reducing the effectiveness of the treatment.”
Instead of viral vectors, Eyevensys uses DNA plasmids encoding therapeutic proteins to treat a variety of retinal diseases, such as wet age-related macular degeneration and geographic atrophy.
Compared with viral vectors, DNA plasmids are less immunogenic, allowing for redosing, and they don’t integrate into the genome, eliminating concerns of mutagenesis. DNA plasmids can also accommodate larger DNA sequences and are relatively easy to manufacture, purify, and produce at scale.
Because DNA plasmids have a lower transfection efficiency than viral vectors, Eyevensys applies electroporation. Specifically, the company uses a proprietary ocular device and pulse generator to help transgenes enter ciliary muscle cells. Within the cells, the transgenes are translated into therapeutic proteins that can reach all parts of the eye. Six months later, the proteins are still found, and side effects are not detected.
A bigger and better biomaterial
Besides physical mechanisms, there are chemical approaches to delivering gene therapy. Chemical approaches that use materials compatible with the human body are less likely to generate immune responses. However, some popular choices, such as lipid nanoparticles, can trigger the immune response as they carry a therapeutic cargo into the cytoplasm.
To address this limitation, Vesigen Therapeutics produces engineered versions of naturally occurring extracellular vesicles called ARMMs (ARRDC1-mediated microvesicles). In the engineered ARMMs, the ARRDC1 protein is attached via a linker to a payload molecule. A variety of payloads may be carried across the cytoplasm without causing immunogenicity.
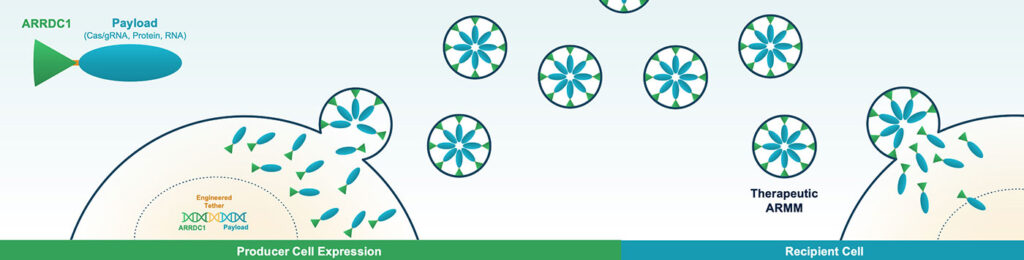
ARMMs have a large payload capacity—large enough to deliver base editing complexes, which are too large for most delivery platforms. Some companies have resorted to splitting base editors so that they may be delivered via separate AAVs and pieced together inside the cell. This significantly increases complexity and cost. ARMMs would likely overcome these challenges, allowing for the delivery of multiple gene editors and gRNA complexes in their fully functional form within a single particle. In addition, unlike viral vectors, ARMMs are transient.
“When you deliver a genome editor, you don’t want it to persist in the cell,” says Joseph Nabhan, PhD, chief scientific officer at Vesigen. “You want that genome editor to be in the cell and the nucleus for a short period of time to edit the target gene and then get degraded. And that’s what virally delivered therapeutics cannot do.”
In vitro tests have demonstrated efficient editing of multiple genomic loci across a range of human and murine cell types and robust modification of the target gene product. Additionally, an in vivo murine study found that a single intranasal administration of ARMMs loaded with gene or base editors targeting immune modulatory genes resulted in 60% or greater on-target editing in macrophages, blunting the release of associated cytokines in lung tissue.
Defying all odds
Another company taking inspiration from a natural mechanism to create a nonviral delivery system is CyGenica. In CyGenica’s case, the natural mechanism is the kind of cell penetration that is accomplished by certain proteins. Such proteins typically have a positive charge and exploit endocytosis. CyGenica, however, has engineered a cell-penetrating protein that has a negative charge and eschews endocytosis.
“When you have a positively charged molecule interacting with a negatively charged cell membrane, it binds strongly and destroys the membrane,” says Nusrat Sanghamitra, PhD, CEO, CSO, and founder of CyGenica. “With our platform, that doesn’t happen because we use a negatively charged molecule.”
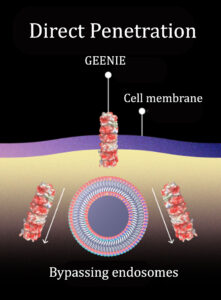
This molecule works like a molecular drill. According to CyGenica, it “deterministically translocates through the cell membrane using a non-endocytic, helical mechanism.”
CyGenica has named its platform GEENIE, which stands for Guided Efficient, and Effortless Navigation. Unlike other delivery platforms, GEENIE directly interacts with the cell membrane and goes through it without making any permanent hole or causing any damage. GEENIE lets the membrane reseal without forming endosomes, which entrap up to 98% of therapeutic molecules.
“Due to the direct cell entry mechanism, GEENIE bypasses the endosomes,” Sanghamitra asserts. “And as opposed to just 2 to 3%, 100% of our platform is available to reach the intracellular target site.”
According to CyGenica, GEENIE is not only effective but also very safe. The company reports that GEENIE has been used to deliver CRISPR gene editing systems into cells without causing any toxicity, and that a high dose (1,800 mg/kg) of the platform in mice resulted in no signs of toxicity or immunogenicity.
CyGenica is currently developing its platform to deliver small-molecule chemotherapy drugs. The company claims that GEENIE can work with any cargo, including RNA molecules, and that it can be made to target any cell type with a known binding partner. Finally, the company says that in vivo studies in mice have demonstrated a good biodistribution of GEENIE throughout the body, including the brain.
Producing a better cell
Ensuring that cell health is maintained is an essential step in the development of therapeutics like gene therapy. One company that takes cell health especially seriously is Avectas, a specialist in the ex vivo delivery of molecules such as mRNA, proteins, and gene editing payloads for cell engineering applications.
“We’re focused on providing a technology for therapeutic developers which produces a gene edited cell with superior health and function compared with existing technologies, creating a more efficacious product,” says Justin McCue, PhD, chief technology officer at Avectas.
The company has developed an ex vivo cell editing technology called Solupore. Inside a closed single-use transfection chamber, cells are engineered by spraying them with a novel solution containing a gene editing cargo as well as an atomizer that creates a transient permeabilization to the cell membrane.
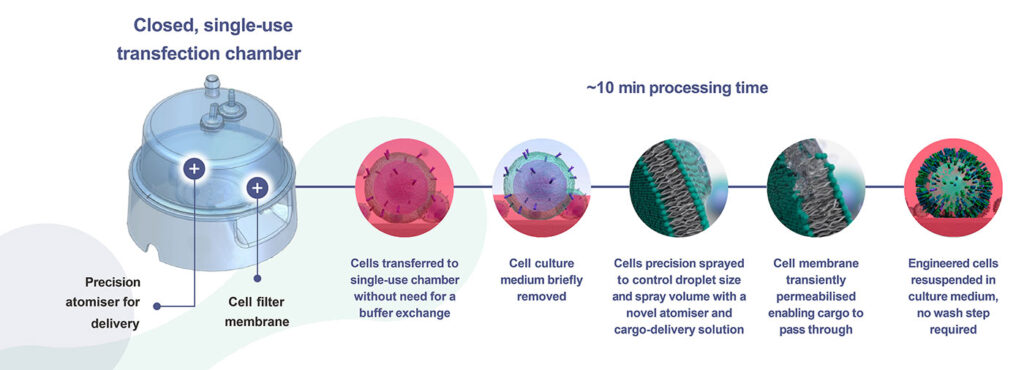
This process takes approximately 10 minutes—a small fraction of a typical cell manufacturing duration. After the process is complete, edited cells are expanded and tests are performed to ensure that quality control and regulatory goals are met. Cells that pass the tests may be introduced to patients.
So far, Solupore has been tested with T cells, natural killer cells, and induced pluripotent stem cells. According to Avectas, in vitro analytical tests—including assays for cell viability, phenotype, apoptosis, avidity, and cytoxicity—have shown that T cells edited using Solupore perform better than cells modified by other methods. The company adds that it used a mouse tumor model to show that in vivo Solupore-edited T cells engrafted better and were more cytotoxic than cells that were produced using existing cell editing technology.
“With the next generation of cell therapy products, we’re seeing an evolution in the industry to produce healthy and more highly functioning cells, potentially reducing the dose requirements based on improved efficacy,” McCue says. “For next-generation cell therapies that require gene modification, Solupore provides the potential to meet that goal.”


