Drug discovery and development companies are racing to bring antibody therapies to market. Competition is fierce to be the first to win FDA approval for first-in-class treatments, especially for cancer.
At the recent Antibody Engineering and Therapeutics conference in San Diego, CA, several new research approaches were presented along with their application in drug development and discovery.
Making therapeutic antibodies means that a living organism, usually a cell line, must manufacture the antibody. Getting the cell lines to do this is generally accomplished by transfection. One company coming up with techniques to shave time off the development timelines for therapeutic antibodies is Maxcyte.
“We offer a platform that transfects cells using Flow Electroporation™ technology to introduce molecules (protein, DNA, or RNA) into a large range of cells—1/2 million to 200 billion. No additional chemicals or viruses are required with the MaxCyte process,” explains James Brady, Ph.D., vice president of technical applications and customer support.
Another advantage of Maxcyte’s systems, according to Dr. Brady, is the ability to generate milligram to gram quantities of protein via transient expression.
“Going to the time and effort of creating stably transfected cells is not necessary when a transient transfection can yield gram quantities of protein,” he says. Systems developed by our company can be used with any cell line, any production process, and any media. This flexibility of our system means that it can be used in a wide variety of experiments needed during the development of antibodies.”
Another issue is the post-translational modification of antibodies. These modifications can vary between cell types used to make the antibodies. Dr. Brady adds that because cells that will be used in manufacturing are easy to employ with the company’s platform, differences in post-translational modification introduced by the use of different cell lines is eliminated. Thus, says Dr. Brady, “data generated on our platform are more relevant.”
Automation Comes to Ab Cloning
At the conference, Kurt Klimpel, Ph.D., field application specialist, product development at SGI-DNA, talked about the synthetic genomics. He said that his company can automate cloning using a DNA genomics workstation, BioXp™ 3200, which permits hands-free, rapid, antibody library construction.
He cited as a case study the antibody engineering group at VIB in Belgium. The group designed and assembled a family of nanobody sequences using the BioXp instead of a traditional manual workflow. The goal of the project was to clone 30 different DNA constructs into a VIB proprietary expression vector.
“The conventional method for doing this involves laboriously designing and ordering primers to build the fragments for cloning into the first vector; transforming this into bacteria, plating, picking clones, using PCR amplify for verification, and finally sequencing for confirmation—and this is only the first half of the project. If all goes well, this takes at least a month of dedicated time for a molecular biologist,” explains Dr. Klimpel.
The project involves submitting the desired DNA sequences. From there, SGI-DNA designs the oligonucleotides and other reagents, sending these reagents on to the customer. The customer then loads the reagents into their BioXpand runs a customized overnight process that both creates the doubled-stranded DNA (dsDNA) Tiles and uses Gibson assembly to clone the de novo dsDNA Tiles into the customer’s desired final vector.
“The customer’s hands-on-time for this process is simply the five minutes it takes to load the reagents and then press the start button. The resulting cloned material is then used for transformation, plating, and sequencing of colonies to verify correctness,” continues Dr. Klimpel.
While the results are not perfect in every case, the accuracy ranges around 85% to 98%, he says, adding that traditional methods take about a month with a dedicated full-time molecular biologist, with the associated overhead and reagents. Using the BioXp in all the steps in this case cut the time from 30 days to 6, Dr. Klimpel points out.
Rapidly Screening Antibodies
Another technical advance discussed at the conference is found with the iQue® screener from IntelliCyt. This screening platform aids in the discovery of new therapeutic antibodies, in understanding the functional efficacy of immuno-oncology therapies, and in screening for highly productive clones during the cell-line generation process, according to the company.
“Our automated platform utilizes a patented rapid sampling technology combined with a flow cytometry engine which very quickly analyzes the cells. Traditional flow cytometry is much slower in comparison and often requires dedicated personnel to run the instrument,” says Thomas Duensing, Ph.D., chief technology officer.
Once the data is gathered, it is rapidly processed to turn the information into actionable results using proprietary software, he explains.
“Core users of our products are researchers looking for therapeutic antibodies. To find [an antibody], they must screen candidates—the quicker the better. The targets for these studies are often molecules expressed on the surface of a cell. These targets can be a receptor or some other kind of signaling molecule,” he says.
Conventional approaches employ ELISAs to screen compounds. This generally only permits one test to be carried out at a time, points out Dr. Duensing, adding that IntelliCyt’s technology permits multiple tests to be carried out at the same time (Figure 1).

“For example, clients use a cell line transfected to express the target. But at same time, the untransfected cell line can be used as a negative control. If the screen is taking place with 10,000 hybridoma clones, then you only have to run it once, rather than twice—this is a huge cost and effort savings,” asserts Dr. Duensing.
In addition to building controls into their primary screens, many customers are using the product’s multiplexing capabilities to screen for cross-reactivity with other species (for example, mouse, rat, monkey, etc.). “This can help when designing downstream experiments in whole animals, because it is already known if the antibody recognizes other species beyond human,” explains Dr. Duensing.
Coding for Therapeutic Antibodies
Treating a patient with antibodies does not always mean that proteins must be manufactured. A promising alternative is using mRNA that codes for an antibody of interest.
“Getting an antibody into humans does not always mean developing and producing the antibody outside the body. Injections of messenger RNA into humans can be used to co-opt a patient’s own cells to make cancer-targeting antibodies. Alternatively, the same approach can be used to induce an antibody response for a vaccine,” says Sebastian Kreiter, M.D., director, Immune Therapy Development Center, vice president of immunotherapy and preclinical research, BioNTech Pharmaceuticals (Figure 2.).
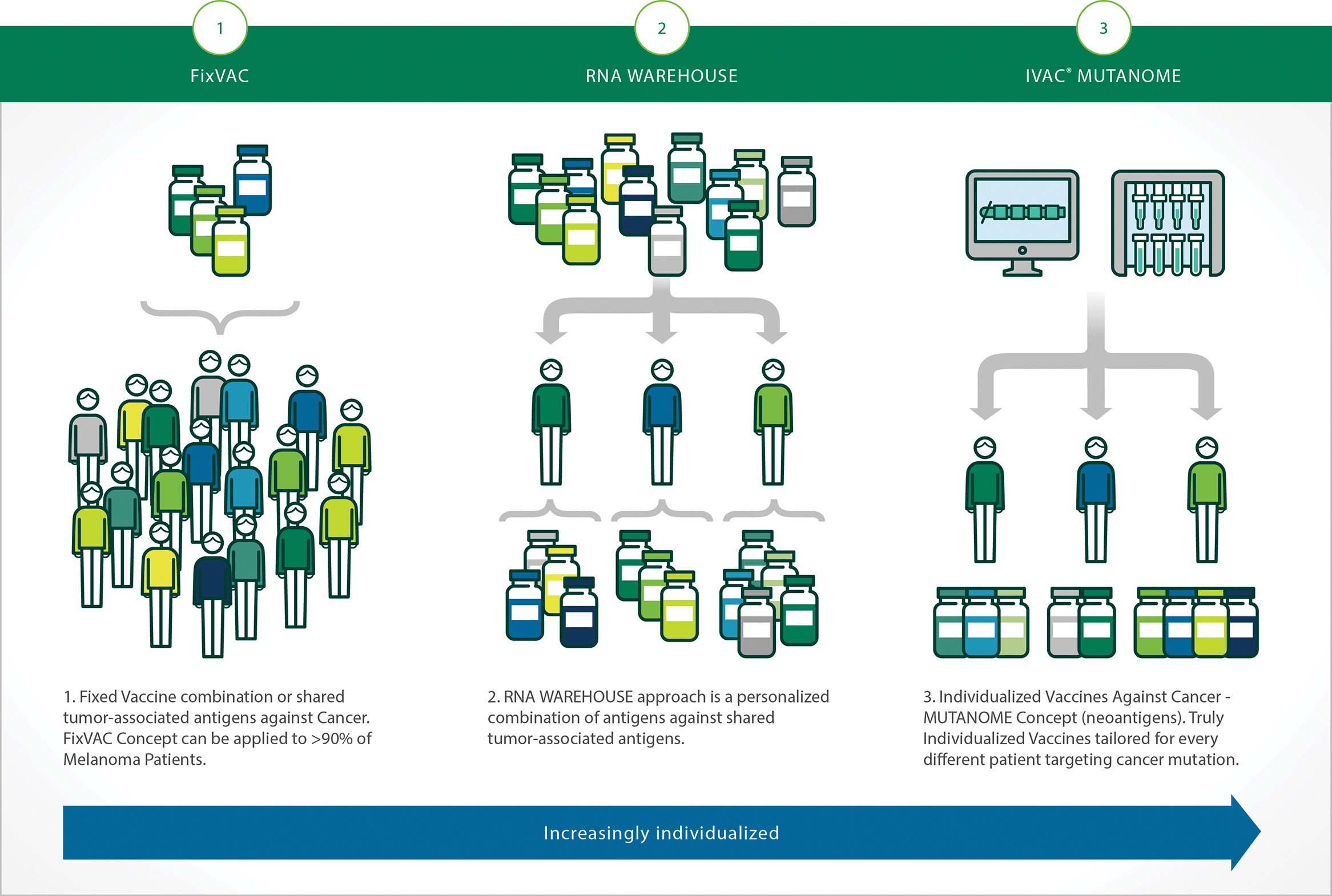
The company has engineered mRNAs to prevent immune reactions of the body. “The final step in purification of therapeutic mRNAs eliminates double-stranded RNA, remaining RNA/DNA hybrids, and impurities,” notes Dr. Kreiter. “These precautions lead to a non-immunogenic mRNA that does not provoke an immune response against the mRNA itself or the encoded protein.”
Preliminary results show that mRNA produced a superior therapeutic outcome when compared to the protein itself, by providing a sustained response. Additionally, by employing mRNA instead of protein, production time for making mRNA is much shorter than that of proteins, with completion of the process done in weeks rather than months, notes Dr. Kreiter, explaining that delivery of therapies via mRNA is a viable alternative to protein-based injections.
“I believe that cancer treatments will be multimodal, not just a combination of small molecule compounds, but a combination of small molecules, either proteins or mRNA, and vaccines,” he says.
Novel Affinity Column Technology
One challenge facing companies is the ability to evaluate the continued efficacy of an immune therapy antibody once the drug has been manufactured and stored. Additionally, the batches of these complex biologics need to have consistent properties, leading companies to search for better and more effective means of determining the activity of stored antibodies. Roche decided to tackle this challenge with the development of the FcRn Affinity Column.
Recall that the Fc portion of a human antibody is the part of the protein that does not vary between IgG antibodies. Furthermore, the receptor for the Fc portion (FcRn) of the antibody is involved in the cellular recycling of the antibody. The constant part of the antibody, not the epitope recognition area, is bound by the FcRn, which then endocytoses it into the cell. After processing the antibody within the cell, the recycled antibody is then moved back to the cell surface, where it is released into the
extracellular space.
Determining Antibody Half-Life
“This recycling of antibodies is the determining factor in the half-life of antibodies in the human body,” explains Tilman Schlothauer, Ph.D., senior principal scientist at the Roche Innovation Center Munich. “Having a simple and inexpensive method to evaluate this characteristic (half-life of an antibody) of a batch of antibodies, or to characterize a new antibody, is a need faced by drug developers and manufacturers.

“Roche has addressed this desire by developing an affinity separation column that binds the Fc portion of antibodies. Conditions in the column closely mimic the physiological interactions of IgG and FcRn.”
Changes such as the oxidation state of methionine residues in the Fc portion could occur during storage. These changes reduce the in vivo half-life of therapeutic antibodies. Two different methionine residues are within the Fc portion of the antibody; with the homodimer of a complete IgG antibody, therefore, four different loci for oxidation are present, resulting in a final number of separate species, based on the oxidation state of the methionine, reaching seven. These species of the oxidized monoclonal antibodies have different retention times in the FcRn separation column.
“When we compare two different antibodies, we find that late-retention times correlate to short pharmacokinetic half-lives of antibodies. This suggests that immunogenic therapies with late-eluting antibodies will have to be more frequently dosed or used with higher doses to be effective,” states Dr. Schlothauer. “We [Roche] see this FcRn column as having very useful applications for extended characterization of drugs, in addition to screening prospective hits before intensive, expensive development.”
DeeAnn Visk Ph.D. Founder and Principal Writer DeeAnn Visk Consulting

