For over three decades, the function of unusual bacterial structures called retrons has remained unclear. Now, a team has solved the longstanding mystery: Retrons are immune system “guards” that ensure the survival of the bacterial colony when it is infected by viruses. In addition to uncovering a new strategy used by bacteria to protect themselves against viral infection—one that is surprisingly similar to that employed by plant immune systems—the research revealed many new retrons that may, in the future, add to the genome-editing toolkit.
This work is published in Cell in the paper, “Bacterial Retrons Function In Anti-Phage Defense.”
The study, conducted in the lab of Rotem Sorek, PhD, full professor, department of molecular genetics, Weizmann Institute of Science, was led by Adi Millman and Avigail Stokar-Avihail, two graduate students and Aude Bernheim, PhD, a postdoc in the lab.
Retrons, found widely in bacteria, are genetic elements comprised of a reverse transcriptase (RT) and a non-coding RNA (ncRNA). The RT uses the ncRNA as a template, generating a chimeric RNA/DNA molecule in which the RNA and DNA components are covalently linked.
Sorek and his team did not set out to solve the retron mystery; they were seeking new elements of the bacterial immune system, specifically elements that help bacteria to fend off viral infection. Their search was made easier by their recent finding that bacteria’s immune system genes tend to cluster together in the genome within so-called defense islands. When they uncovered the unique signature of a retron within a bacterial defense island, the team decided to investigate further.
Their initial research showed that this retron was definitely involved in protecting bacteria against bacteriophages—viruses that infect bacteria. As the researchers looked more closely at additional retrons located near known defense genes, they found that the retrons were always connected—physically and functionally—to one other gene. When either the accompanying gene or the retron was mutated, the bacteria were less successful in fighting off phage infection.
The researchers then set out to look for more such complexes in defense islands. Eventually, they identified some 5,000 retrons, many of them new, in different defense islands of numerous bacterial species.
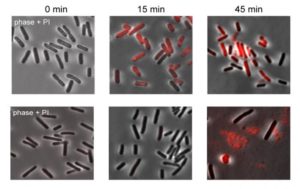
How do retrons do this? Focusing back on one particular kind of retron and tracing its actions in the face of phage infection, the research team discovered that its function is to cause the infected cell to commit suicide. Cell suicide, once thought to belong solely to multicellular organisms, is a last-ditch means of aborting widespread infection—if the suicide mechanism works fast enough to kill the cell before the virus finishes making copies of itself and spreading out to other cells.
Further investigation showed that retrons do not sense the phage invasion itself, but rather keep watch on another part of the immune system known as RecBCD, which is one of the bacterium’s first lines of defense. If it realizes that the phage has tampered with the cell’s RecBCD, the retron activates its program through the second, linked genes to kill the infected cell and protect the rest of the colony.
“It’s a clever strategy, and we found it works in a similar way to a guard mechanism employed in plant cells,” says Sorek. “Just like viruses that infect plants, phages come equipped with a variety of inhibitors to block assorted parts of the cell immune response. The retron, like a guard mechanism known to exist in plants, does not need to be able to identify all possible inhibitors, it just has to have a handle on the functioning of one particular immune complex. Infected plant cells apply this ‘abortive infection’ method, killing off a small region of a leaf or root, in an effort to save the plant itself. Since most bacteria live in colonies, this same strategy can promote the survival of the group, even at the expense of individual members.”
Retrons are useful to biotechnology because they begin with a piece of RNA, which is the template for the synthesis of the DNA strand. This template in the retron sequence can be swapped out for any desired DNA sequence and used, sometimes in conjunction with another tool borrowed from the bacterial immune toolkit—CRISPR—to manipulate genes in various ways. Sorek and his team believe that within the diverse list of retrons they identified may be hiding more than a few that could provide better templates for specific gene editing needs.