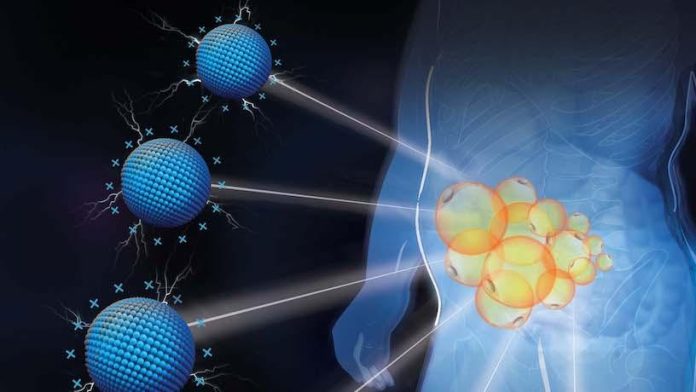
Two studies reported separately by researchers at Columbia Engineering and Columbia University Irving Medical Center (CUIMC) demonstrate a new approach to obesity treatment that uses a positively charged cationic nanomaterial, PAMAM generation 3 (P-G3), to target specific areas of visceral and subcutaneous fat, and inhibit the unhealthy storage of lipids in enlarged fat cells, effectively shuts off the lipid storage function of adipocytes. In contrast with strategies such as liposuction that remove or destroy fat, the new nanomaterial works to remodel the fat. The team said the approach may represent a way of targeting fat cells depot-specifically and healthily.
“Our approach is unique; it departs from the pharmacological or surgical approaches,” noted research co-lead Li Qiang, PhD, associate professor of pathology and cell biology at CUIMC, who specializes in obesity and adipocyte biology. “We used cationic charge to rejuvenate healthy fat cells, a technique no one has ever used to treat obesity. I think this novel strategy will open the door to the healthier and safer reduction of fat.”
Qiang further told GEN, “We are definitely interested in developing this exciting approach into unprecedented treatment to local obesity, being a more specific, aesthetic purpose for subcutaneous fat, and a therapeutic strategy for treating visceral obesity.” The aim will be to engineer and screen for different formulations of cationic materials that might display even better efficacy and safety, “though we are pretty happy with the safety and efficacy of our current formulations,” GEN learned. “We will also test delivering fat-manipulating drugs and genes to achieve additive improvements.”
One of the team’s newly reported studies, published in Nature Nanotechnology and titled “Selective targeting of visceral adiposity by polycation nanomedicine,” focuses on visceral adiposity, or belly fat. The other study, published in a report in Biomaterials, and titled “Polycationic PAMAM ameliorates obesity-associated chronic inflammation and focal adiposity,” describes the use of nanomaterials to target subcutaneous fat as well as chronic inflammation associated with obesity.
“Obesity and being overweight are “surging global health challenges, inflicting severe comorbidities including diabetes and cardiovascular diseases to account for the second most preventable death,” Qiang and colleagues noted in their Nature Nanomaterials paper. “Over the past half-century, their rates have tripled and will threaten over 2.5 billion adults by 2025,” they continued in their paper in Biomaterials. “The high prevalence is particularly alarming given that obesity and overweight predispose affected individuals to a number of serious comorbidities, such as type 2 diabetes mellitus (T2DM), cardiovascular diseases, osteoarthritis, and various cancers.
The development of fat cells, which are produced from a tiny fibroblast-like progenitor, not only activates the fat cells’ specific genes but also grows them by storing more lipids (adipocytes and adipose tissue). In fact, lipid storage is the defining function of a fat cell. “Obesity is directly caused by the expansion of white adipose tissue (WAT), owing to the formation and growth of adipocytes,” the team continued in their Nature Nanotechnology report. “Adipocytes function by storing lipids in the form of triglycerides (TGs).” In fact, they noted, the size of an adipocyte can grow up to 20-fold in diameter, theoretically holding ~8,000-fold more lipids.
The ability to target fat cells and safely uncouple unhealthy fat formation from healthy fat metabolism would be the answer to many peoples’ prayers. A major challenge in obesity treatment is that fat tissue, which is not continuous in the body but is found piece by piece in “depots,” has been difficult to target in a depot-specific manner, pinpointed at the exact location.

To date, there has been no way to specifically treat visceral adipose tissue. And current treatments for subcutaneous fat like liposuction are invasive and destructive. “ … obesity treatment remains an unprecedented challenge, particularly for visceral adiposity,” the investigators further noted.
The scientists, headed by Qiang, and research co-lead Kam Leong, PhD, the Samuel Y. Sheng professor of biomedical engineering and of systems biology at CUIMC, recognized that adipose tissue contains large amounts of negatively charged extracellular matrix (ECM) to hold fat cells. They thought that this negatively charged ECM network might provide a highway system of sorts for positively charged molecules. So they took a positively charged nanomaterial, P-G3, and injected it into diet-induced obese (DIO) mice. P-G3 quickly spread throughout the tissue and the team found that this method of specifically targeting visceral fat worked.
“The original purpose of this study was to find some approach to target fat depot specifically,” Qiang explained to GEN. “Cationic nanomaterials have been extensively studied in Dr. Leong’s lab for tackling various inflammatory diseases ranging from sepsis to inflammatory bowel disease and to cancer. These cationic biomaterials seem to provide a therapeutic benefit to these diseases in animal models but have never been tested in treating obesity. We initially found they preferentially deposited in adipose tissue, giving us the hope of depot-specific targeting of fat to fulfill a critical gap in the obesity field.”
The two studies also showed that the cationic P-G3 nanomaterial exhibited an intriguing ability. While it helped new fat cell formation, it also uncoupled lipid storage from the housekeeping functions of fat cells. And this inhibition of unhealthy lipid storage in enlarged fat cells resulted in more metabolically healthy, young, small fat cells in the treated mice, more akin to those found in newborns and athletes. The P-G3 effectively shut off the lipid storage program in fat cells so that the treated mice lost weight. This was totally unexpected, given the well-established function of P-G3 in neutralizing negatively charged pathogens, such as DNA/RNA cell debris, to alleviate inflammation.
“Intraperitoneal [IP] delivery of P-G3 alleviated the chronic inflammation in DIO mice and reduced their body weight, resulting in improved metabolic functions,” the team noted in their Biomaterials report. And commenting on their results in the Nature Nanomaterials paper, they wrote, “Here we demonstrate that the polycation P-G3 is selectively distributed to visceral fat via the i.p. delivery route to inhibit visceral adiposity and prevent obesity by uncoupling lipid synthesis from adipocyte development, creating ‘dwarf’ adipocytes.”
So while the nanomaterial’s preferential targeting of fat cells offers up the potential to deliver drugs to fat locally, the “huge surprise, in a good way,” Qiang reiterated, was that the cationic nonmaterial itself caused a strong reduction of fat, without the need to add drugs. “Cationic nanomaterials have the same effect on visceral fat (bad fat) and subcutaneous fat (good fat) once reaching them,” he told GEN. “The nanomaterials can make the fat cells smaller and healthier,” by impacting lipid storage, but without impacting the adipocytes’ other functions, resulting in ‘dwarf’ adipocytes. We just need to make sure the nanomaterials can be delivered into visceral or subcutaneous fat as we want.”
The researchers found that this uncoupling function of P-G3 also held true in human fat biopsies, signifying the potential of translating their technology to humans. “With P-G3, fat cells can still be fat cells, but they can’t grow up,” said Leong, a pioneer in using polycation to scavenge pathogens. “Our studies highlight an unexpected strategy to treat visceral adiposity and suggest a new direction of exploring cationic nanomaterials for treating metabolic diseases.”
Now that they can selectively target visceral fat, Leong and Qiang envision several applications. The Biomaterials-published study demonstrated a simple approach that could be used for aesthetic purposes. Similar to Botox, P-G3 might be locally injected into a specific, subcutaneous fat depot. The investigators, who have patents pending, are now engineering P-G3 into derivatives to improve efficacy, safety, and depot specificity.
Qiang suggested to GEN that aesthetic applications of the technology to reduce focal subcutaneous fat could be realized within a couple of years, although it will take some additional years to develop a treatment for visceral fat. Moreover, Qiang suggested, there seems to be no major challenge in translating the technology into a treatment for subcutaneous fat. “We already have a good formulation, and its precise local distribution maximizes the efficacy and minimizes the toxicity,” he pointed out. “The nanomaterials have been tested in human fat biopsies and worked well, so we are confident that we can replicate the results in larger animal models before starting clinical trials.”
For applications against visceral obesity, additional work will be needed, particularly in areas such as safety, Qiang commented. “So far we have only studied in detail the intraperitoneal injection as the delivery route. It is practical and common in the clinic, but may still be considered a bit invasive. Can we find a better delivery route? We’ll try.” One potential way of improving the technology will be by increasing treatment intervals—from weekly, to monthly, for example—which is one avenue of research that the team will be pursuing.
The researchers are also particularly encouraged by the potential to develop P-G3 into a platform that can deliver drugs and gene therapies specifically to a given fat depot. This may repurpose many drugs for which there are systemic safety concerns, such as thiazolidinediones (TZDs), a potent but unsafe type of drug that is a strong modulator of fat and used to treat type 2 diabetes—but which has been linked to heart failure and banned in several countries. “Collectively, our study highlights a strategy to target visceral adiposity and suggests cationic nanomaterials could be exploited for treating metabolic diseases,” the team commented in the Nature Nanotechnolgy report. In their paper in Biomaterials they further concluded, “Using P-G3 to deliver fat-manipulating reagents and gene therapies into a targeted location may achieve the additive benefit of inhibiting adiposity. Further, the strong suppression of the lipogenesis program by P-G3 may facilitate the discovery of novel mechanisms to manipulate adipocytes.”
Qiang added, “We’re very excited to discover that cationic charge is the secret to targeting adipose tissue … Now we can shrink fat in a depot-specific manner—anywhere we want—and in a safe way without destroying fat cells. This is a major advance in treating obesity.”