Neuronal inflammation is the common denominator in diseases such as Alzheimer’s disease, Parkinson’s disease, Multiple Sclerosis, Age-Related Macular Degeneration and Type 2 Diabetes, that have multiple risk factors but whose precise causes remain unknown.
Inflammasome Therapeutics, a private company in Newton, MA, founded by Jayakrishna Ambati, MD, and Paul Ashton, PhD, in 2016, is committed to developing therapeutics that target inflammation and drug delivery systems that release the active ingredient over extended periods.
The company has licensed Kamuvudines—a class of molecules that successfully inhibit inflammasome activation in cell culture and animal models—and is poised to go into clinical trials early next year.
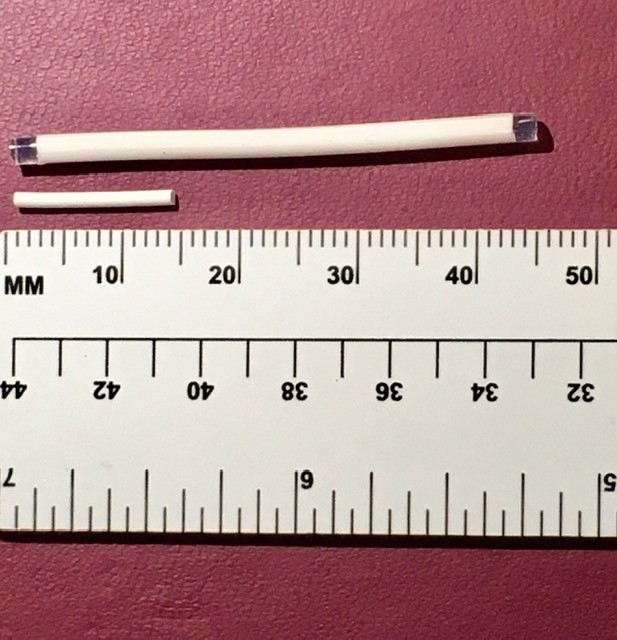
Inflammasome Therapeutics established an up-to-$160 million collaboration with Boehringer Ingelheim in 2019 to develop therapies for retinal degenerative diseases. In 2020, the company received a $1 million grant from the Bill and Melinda Gates Foundation to develop a bioerodible implant for birth control that accomplishes a steady release of the contraceptive over 18 months followed by dissolution of the implant, precluding the need for its surgical removal.
The company has recently been awarded another $1.3 million grant from the Gates Foundation to develop dual bioerodible sustained-release implants that combine drugs for HIV prevention and birth control. The implant will deliver a steady dose of islatravir for HIV prevention and the hormone levonorgestrel for birth control. Once the drugs have been completely released over the span of a year, the implant is designed to erode.
HIV is the leading cause of death among women of reproductive age making this technology an asset in reducing female morbidity and mortality. Females account for 58% of the estimated 240,000 new HIV infections in Western and Central Africa. An implant that dissolves once it has served its purpose will be particularly beneficial in developing countries where it is difficult to maintain follow-ups with recipients. The $2.6 billion global HIV prophylaxis market was dominated by daily oral medications in 2019.
The team at Inflammasome is refining the sustained release technology which can be optimized for the delivery of almost any small molecule requiring consistent dosing over an extended period.
GEN Edge interviewed Ashton, Inflammasome’s CEO and the inventor of four FDA-approved drugs, about the company’s strategy, its reasoning behind targeting inflammation, and its promising pipeline of products based on the sustained-release technology for drug delivery.
GEN Edge: What are the big goals and priorities of Inflammasome Therapeutics over the next 1–2 years?
Paul Ashton, PhD: We are planning to start clinical trials with two products next year—our own products for geographic atrophy and multiple sclerosis. At the same time, we are working with our collaborations. One is with Boehringer Ingelheim. The other is with the Gates Foundation on two projects—one a bioerodible birth control implant and the other is an implant that releases both the birth control and an anti-HIV compound to prevent transmission of HIV. The program with Boehringer Ingelheim is in ophthalmology.
GEN Edge: What drugs suppressing inflammasome activation are in the pipeline at Inflammasome Therapeutics?
Ashton: This came out of work that was published in Science in 2014 by one of the co-founders of the company, Jay Ambati. He showed that inflammasome activation is extremely important for the progression of geographic atrophy. That has subsequently been corroborated by other investigators.
GEN Edge: What is geographic atrophy?
Ashton: Age-related Macular Degeneration (AMD) comes in two types. The first and fortunately treatable type is called “wet” AMD. It is characterized by leaky blood vessels that eventually hemorrhage and cause blindness. That is treated by injections of antibodies against VEGF. Lucentis, Avastin and things in that nature. That affects about 15% of people with geographic atrophy.
The rest are affected by a form called “dry” AMD. It’s characterized by atrophy—a slow death of the cells that lie under the retina, retinal pigmented epithelial (RPE) cells. And as they die, they unfortunately take the overlying retina with them. In the more severe cases, you see this big blotch appearing on the retina, which is a geography of atrophy, hence geographic atrophy. You can see it when you image the eye.
Dry AMD unfortunately is untreatable right now. A couple of companies are in Phase III clinical trials with complement inhibitors. Let’s hope they work. It turns out that when you look at the pathological samples of eyes with geographic atrophy—absolutely you can find different forms of complements in them–but you’ll also find amyloid beta that we all recognize from Alzheimer’s, tau tangles, iron overload, and Alu RNA.
Symptomatic patients have all these factors in the eye. And the concern is if you take out any one of them—that might be the one that is driving everything, but equally it could be that the disease shrugs and goes back to work with all the other factors.
The one fascinating thing that has become apparent over the last 10 years is that all these factors upregulate inflammasome activation that go on to make IL-1beta, IL-18 etc. We think if you can inhibit inflammasome activation, you should be able to have a significant effect on geographic atrophy.
I could make the same argument for Alzheimer’s disease, Parkinson’s disease, or Multiple Sclerosis because they are all neuroinflammatory diseases. These are multifactorial diseases that all progress by inflammasome activation.
In 2014, Jay published a paper showing, shockingly, that some existing drugs, nucleoside reverse transcriptase inhibitors (NRTIs)—things like AZT—naturally inhibit inflammasome activation. In all models we have known subsequently, they are very effective. You can inject complement under the retina, and you get a model of geography atrophy. This will stop that.
You can inject Alu RNA or all the other factors. They can all be blocked with NRTIs. My challenge to Jay when he first told me this was, what is the incidence of geographic atrophy in people who have been taking NRTIs for a long time?
Now with big data you can crawl through enormous databases. When Jay did that, he found that in the patients who were taking NRTIs as pre-exposure prophylaxis (PREP), there is about a 3–4-fold reduction in the risk of developing geographic atrophy compared to the age, weight and disease-matched control population. The p value is tiny because you’re dealing with such enormous sample-sizes. That was published earlier this year in PNAS. We now have a class of drugs that we “know” works in preventing geographic atrophy in humans and also in a very dramatic severe animal model.
The problem with NRTIs is they’re toxic. They all have black box warnings. You take them for HIV. It turns out that the toxicity of these compounds comes from phosphorylation. The phosphorylated form is effective against HIV. Unfortunately, they also act against mitochondria which is the basis for their toxicity. If you block their phosphorylation, say with a methyl group, you have a compound that has no activity against HIV, has no mitochondrial toxicity, but retains the anti-inflammasome activity.
We have a suite of compounds that seem extremely effective in our geographic atrophy preclinical models and–what isn’t published yet—also effective in multiple sclerosis models. We have picked one whose structure lends itself for intraocular delivery. This is the other half of the company which is about implants. We can deliver it into the eye for a long time with a single injection. That is the product we are looking to take into the clinic shortly.
On the other side, we have another compound that is another one of the Kamuvudines—chemical derivatives of NRTIs that have more potent anti-inflammasome activity than the parent molecules yet cannot undergo the same metabolism and have no detectable mitochondrial toxicity—that appears to have good blood brain, blood eye penetration after systemic administration.
GEN Edge: In blocking systemic inflammation—an innate immune response needed in the body—do you find any adverse effects in these different disease models?
Ashton: I can only say I am not aware of any anti-inflammatory problems associated with the parent compounds, NRTIs. And we have decades of history of people taking these drugs. Possibly when you do a clinical trial you may find you’re more susceptible to some infections. Until you do a large clinical trial, you won’t know the extent of those issues that may or may not arise. One of the interesting things is that inflammation is upregulated as we age.
The jury will be out till the clinical trials but we’re very comfortable. Certainly, there inevitably will be side effects with every drug. Then we’re talking about risk and reward. One of the nice things about the implants we are developing for geographic atrophy is that they will be injected directly into the eye. So, there will be virtually no systemic exposure.
GEN Edge: What are your thoughts behind targeting inflammation which is an effect and not the cause in these varied diseases? How do you think targeting effects versus causes balance in the drug development?
Ashton: I’ll take a direct example, Behçet’s Disease—a genetic issue where people develop uveitis that can be blinding. Treatments can be problematic. It’s difficult to get drugs into the eye even if you can avoid systemic inflammation. We originally developed the Retisert device which was a surgical implant that released the steroid drug into the eye. Now it is being sold as Yutiq which is an injection of a steroid into the eye.
Yes, absolutely we are treating the symptoms. We are not changing the genetic makeup. But who cares? I remember one of the early patients was a kid in Israel. This poor kid had been on high-dose systemic steroids for several years. He had a couple of spontaneous fractures due to osteoporosis, was quite hairy, and had the moon face of classic Cushing Syndrome. On top of that his vision was lousy. He got an implant in each eye and his vision improved to about 20–40. He could taper off the huge doses of steroids he was being given and he was entirely happy.
We don’t know what triggers Alzheimer’s disease. But once the disease is running away, and inflammatory cytokines are being produced, it is self-sustaining. The ball is rolling down the hill. Maybe we don’t know enough yet about the people who have elevated amyloid beta who don’t have neurological problems. Certainly, inflammation is exacerbated in the situation. So maybe if you take that out and control it, you can slow down the rate of damage to such an extent that it doesn’t really matter.
We plan to start clinical trials early next year for geographic atrophy and in multiple sclerosis.
GEN Edge: What caused you to shift focus from targeting inflammation to the developing sustained release implants for the release of contraceptives and HIV drugs?
Ashton: The team here at Inflammasome is experienced in making implantable drug delivery systems. We have developed four FDA approved drugs, all of which were implantable drug delivery systems. We have a collaboration with Boehringer Ingelheim to make ocular implants. We already have this path going forward.
In chatting with the folks at the Bill and Melinda Gates Foundation, they expressed an interest in making bioerodible implants to deliver the hormone levonorgestrel for birth control with the idea of lasting a certain period. It should be easy to insert. That’s something I believe we could do readily and easily.
The next request from Gates was to make an implant that also released the new and potent compound for HIV, EFdA (Islatravir). It is under development as an oral drug and hasn’t been FDA approved yet.
It is now feasible to have an implant that would release the drug for a reasonable period. We’re aiming for 12–18 months. So, a combination of those two things would be extremely effective—certainly in many developing countries. You can then make a simpler version that releases merely EFdA that would potential be an HIV prophylactic for everybody.
GEN Edge: What is the significance of combining the HIV drug and the birth control hormone?
Ashton: It would be very beneficial for women who are concerned about unplanned pregnancies and about potential HIV infections. This would be a single injection that would accomplish both of those goals. With any oral drug you have the potential to forget to take it. And inevitably you’re going to need a higher dose where you’re not worried about oral bioavailability and first-pass effects.
What these technologies allow you to do is to maintain a very constant steady level of the drug in the blood. A lot of times you will get side effects due to an initial peak in the concentration. Also, it is wasteful to have too much drug initially. The idea is to have a nice steady concentration that’s above the therapeutic target you’re aiming for.
GEN Edge: What is the scientific basis of being able to accomplish a constant steady release of a drug instead of initial or intermittently bursts?
Ashton: It all depends on the characteristic of the drug. There’s no magic device that will deliver a particular drug indefinitely. You have to tweak and adjust the design of the device to match the physical and chemical properties of the drug that you are trying to deliver. Over the last twenty odd years, our team has become very adept at doing that.
GEN Edge: Your recent press release says this technology may be applied in the sustained release of “almost any small molecule.” What is the scientific basis this technology’s versatility?
Ashton: The basic idea is that you can compress your drug into a core and coat the core in an impermeable polymer layer with a small diffusion port. The size of the diffusion port will control the rate at which the drug is released. If you do it carefully, if the drug is insoluble enough, and if the port is small enough, you will have an equilibrium set up within the device such that the concentration of the drug in the device is more or less saturated. When that occurs, the diffusion gradient across the port is going to be constant. So, the release rate is going to be constant over a prolonged period.
GEN Edge: And is the bioerodible nature of the device since the polymer itself dissolves over time?
Ashton: That’s correct. People have typically embedded drugs in matrices of bioerodible polymers such that the drug is released as the polymer dissolves. The trouble with that is that the shape of the device changes over time causing the release rate to decrease as the whole thing gets smaller. The other problem is that drugs will inevitably try to leach out of the polymers that are being used. The leaching effect typically also gives you simple first order kinetics, so start off fast slows down.
Years ago, a group from Bob Langer’s lab in MIT came up with bioerodible wafers. The idea behind that is that you have a big surface area that doesn’t change much over time. That’s okay if you have a big open space into which you can implant a wafer—(I think the technology was used in patients following surgery to remove a brain tumor—there you’ve got a big space)—but not so practical if you’re trying to inject something.
GEN Edge: What is the advantage of having an injectable implant over a subcutaneous or dermal implant?
Ashton: Most people would rather have an injection than an incision to insert a device. Certainly, for the birth control and HIV combination products. You don’t want to be making an incision and putting something in, closing it up, and then x number of years later the patient has to come back to remove it.
The current products on the market for birth control—certainly Jadelle and Norplant—start off with a quick release rate that generally causes side effects. They achieve therapeutic levels for a certain period then they drop off so that they are below therapeutic levels. Then you have a questionable efficacy in birth control. Potentially after x number of years, perhaps the woman wants to have children. Either way you must go back and remove the implant. And they can potentially break as they are being removed. It takes an incision to take it out and then you’re digging around the incision site trying to make sure you get the things out. That’s not ideal. It would be much better if they just eroded.
For us the key is that they retain the shape while they are delivering the drug. Once the drug’s gone, then they erode. People have looked at bioerosion to deliver drugs. But our view is to use it to get rid of used up devices. Particularly in the eye, where you don’t want the back of the eye to become a graveyard of used up devices.
GEN Edge: What are the technical challenges and opportunities in sustained-release technology? What do you see as your competition?
Ashton: For ocular drug delivery, a lot of people are trying to make ocular implants. They have the same problems—typically they start off releasing the drug very quickly and then they slow down. That’s not optimal. No doubt there will be people who come up with new devices. One would hope so.
I think we have a good patent position on the drugs that we’re delivering, and we have a novel delivery system that ultimately works. That’s really the gist of it.
In developing implants for developing nations, it is a bit of a technical challenge. To give an example, ILUVIEN®, which is an implant that I developed with a company called Alimera Sciences, and YUTIQ®, are sold in the U.S. for about $8000 each. That’s a lot of money to do some clever technology with. To develop something for the developing nations, you are trying to make it for 40 bucks, all in. That introduces some of its own challenges. You have to do something that is going to be easy to manufacture in addition to the other technical things. But I think we’re doing this for long enough now to have most of these things sorted out. I’m not too bothered about the technical challenges. There are challenges in socioeconomic distribution and education to be sure which are for the Gates Foundation to figure out. We’ll just make the implants.
GEN Edge: Is there anything else you’d like to add?
Ashton: With Boehringer Ingelheim, it is an up-to-$160 million collaboration, which has helped establish the company. The Gates Foundation money is less but every bit is important. I’m really looking forward to the EFdA birth control implant.
Anjali Sarkar PhD is a science editor with GEN.