Harmful and healing immune responses to cerebrovascular injury depend on the timing of the response and cells mediating the response, a new study reports.
Injury to blood vessels in the brain, or cerebrovascular injury, commonly caused by a stroke that blocks a blood vessel (ischemic stroke), can result in severe swelling and inflammation.
In patients with short-term blockade of a blood vessel in the brain removing a blood clot from the lumen of a prominent blood vessel to restore blood flow, is often associated with poor clinical outcomes.
To investigate the reasons behind this, scientists at the National Institute of Neurological Disorders and Stroke (NINDS) modeled blood vessel injury of the brain in mice, applying ultrasound directly on the brain, and monitoring local and systemic myeloid cells in space and time, to better understand the underlying mechanisms.
The study reports, specific immune cells in the brain that ultimately help with brain repair, can lead to fatal swelling immediately after an injury, suggesting that timing may be critical when administering treatment after cerebrovascular injury.
The findings are published in an article titled, “Temporally Distinct Myeloid Cell Reponses Mediate Damage and Repair After Cerebrovascular Injury” in the journal Nature Neuroscience and was funded by the NINDS Intramural Research Program at NIH.
“Fixing the brain after injury is a highly orchestrated, coordinated process, and giving a treatment at the wrong time could end up doing more harm than good,” said Dorian McGavern, PhD, NINDS scientist and senior author of the study.
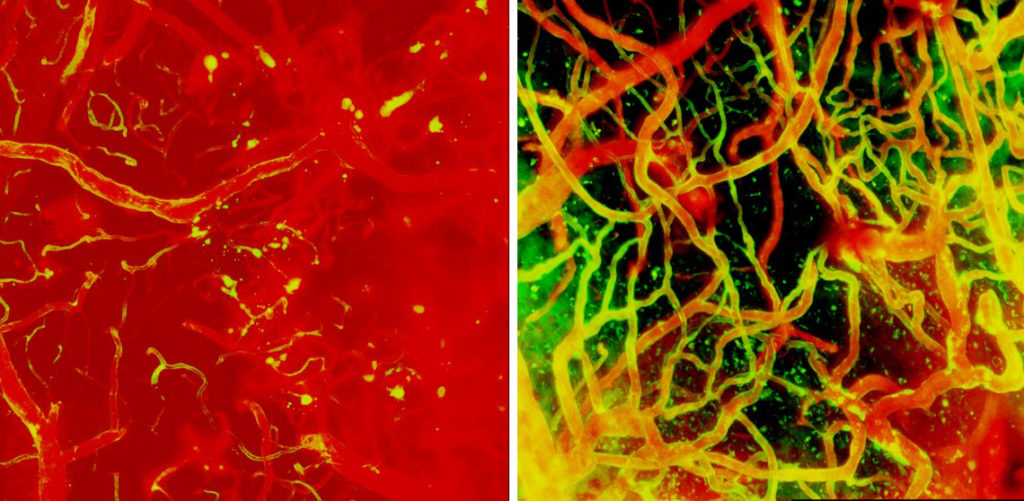
“Timing is of the essence when trying to prevent fatal edema. You want to prevent the acute brain swelling and damage, but you do not want to block the monocytes from their beneficial repair work,” says McGavern.
McGavern and his team use microscopic imaging to analyze how the mouse brain responds to cerebrovascular damage in real-time, with the goal of identifying new therapeutic strategies. Immediately after injury, brain immune cells known as microglia rapidly move to stop blood vessels from leaking by wrapping around broken blood vessels to plug any holes and reinforce their walls. McGavern and his group discovered that inadvertently removing microglia, along with an obstructing clot, causes irreparable bleeding and damage in the brain.
Other circulating immune cells such as peripheral monocytes and neutrophils, including myelomonocytes, that arrive at the site of damage a few hours later, perforate blood vessels as they rapidly move from the circulation into the brain tissue, causing fluid to enter the brain that leads to edema.
“The myelomonocytic cells at this stage of repair mean well, and do want to help, but they enter the brain with too much enthusiasm. This can lead to devastating tissue damage and swelling, especially if it occurs around the brain stem, which controls vital functions such as breathing,” says McGavern.
Once the initial enthusiasm of immune cells infiltrating the damage-site has dampened, monocytes—another type of immune cells that engulfs detritus—enter the brains at a restrained rate and repair the damaged vessels together with designated microglia (repair associated microglia, RAMs). Together these cells rebuild the damaged network of blood vessels in under a couple of weeks.
McGavern and his team go on to test a therapeutic strategy that curbs edema upon brain injury. They show that treatment with antibodies that block myelomonocytic cells from entering the brain by neutralizing two different molecules that help these cells adhere to the wall of the blood vessel at the site of damage prevents swelling if administered within six hours of the injury.
The authors also show that the therapeutic antibodies do not work if given after the six-hour window or if they were given for too long. In fact, treating mice over a series of days with these antibodies inhibits the proper repair of damaged blood vessels, leading to neuronal death and brain scarring.
These findings are currently slated for clinical trials and further studies will examine the intricacies of cerebrovascular repair to identify other therapeutic targets.


