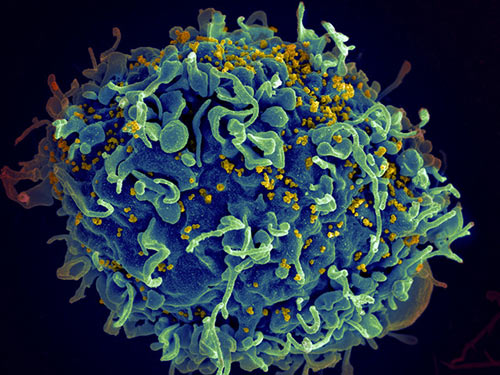
A research team led by scientists at the University of Utah (U of U) Health and the Rockefeller University has determined how a genetic mutation found in mice and in some New World monkeys interferes with how viruses such as HIV and Ebola infect cells. The gene, called RetroCHMP3, encodes an altered protein that disrupts the ability of certain viruses to exit an infected cell, and so prevents it from going on to infect other cells. The researchers suggest that the finding could help inform the future development of strategies for human therapeutics.
“This was an unexpected discovery,” said Nels Elde, PhD, senior author of the study and an evolutionary geneticist in the department of human genetics at U of U Health. “We were surprised that slowing down our cell biology just a little bit throws virus replication off its game.”
The team reported its findings in Cell, in a paper titled, “RetroCHMP3 blocks budding of enveloped viruses without blocking cytokinesis.”
In humans and other animals, a protein called charged multivesicular body protein 3, or CHMP3, is well known for playing a key part in cellular mechanisms that are vital for maintaining cellular membrane integrity, intercellular signaling, and cell division. “The endosomal sorting complexes required for transport (ESCRT) pathway mediates essential cellular membrane fission events such as multivesicular body formation, cytokinetic abscission, and resealing of the post-mitotic nuclear envelope,” the authors explained. Some viruses, including HIV, which are known as enveloped viruses, hijack this ESCRT pathway to exit infected cells, which they do by encasing themselves in the cell membrane and then budding off from the host cell.
The new study has found that the variant version of CHMP3, known as RetroCHMP3, which is found in monkeys and mice, delays that process long enough that the virus can no longer escape. RetroCHMP3 originated as a duplicated copy of CHMP3. So while humans only have the original CHMP3, species such as monkeys, mice, and other animals, have retroCHMP3 or other variants.
Based on their research, Elde and his colleagues suspected that the duplications of CHMP3 that they discovered in primates and mice blocked the ability of enveloped viruses to co-opt the ESCRT pathway into their escape mechanism, as protection against viruses like HIV and other viral diseases.
Building on their hypothesis, Elde and other scientists began exploring whether variants of CHMP3 might work as an antiviral. In laboratory experiments conducted elsewhere, a shorter, altered version of human CHMP3 successfully prevented HIV from budding off cells. There was, however, a glitch: the modified protein also disrupted important cellular functions, causing the cells to die.
Unlike other researchers, Elde and his colleagues at U of U Health had naturally occurring variants of CHMP3 from other animals available. So, working in collaboration with Sanford Simon, PhD, co-author and professor of cellular biophysics at the Rockefeller University, along with Phuong Tieu Schmitt, PharmD, research associate and Anthony Schmitt, PhD, professor of molecular virology, both at Pennsylvania State University, they tried a different approach.
Using genetic tools, they coaxed human cells to produce the version of retroCHMP3 found in squirrel monkeys. When they then infected the cells with HIV, they found that the virus had difficulty budding off from the cells, essentially stopping them in their tracks. “When expressed in human cells, these retroCHMP3 proteins potently inhibit the release of retroviruses, paramyxoviruses, and filoviruses,” the investigators wrote. And this occurred without disrupting metabolic signaling or related cellular functions that can cause cell death. “Remarkably, retroCHMP3 proteins have evolved to reduce interactions with other ESCRT-III factors and have little effect on cellular ESCRT processes, revealing routes for decoupling cellular ESCRT functions from viral exploitation,” the team noted.
The scientists also suggested that an antiviral approach based on exploiting retroCHMP may prove more durable than existing antiviral strategies. “Additionally, the observation that retroCHMP3 alters ESCRT pathway function instead of targeting a viral protein raises the intriguing possibility that retroCHMP3 may be more resistant to viral counter-adaptations than other antiviral proteins that directly inhibit viral replication,” they stated.
“We’re excited about the work because we showed some time ago that many different enveloped viruses use this pathway, called the ESCRT pathway, to escape cells,” said Wes Sundquist, PhD, co-corresponding author of the study and chair of the department of biochemistry at the University of Utah. “We always thought that this might be a point at which cells could defend themselves against such viruses, but we didn’t see how that could happen without interfering with other very important cellular functions.”
From an evolutionary perspective, Elde believes this represents a new type of immunity that can arise quickly to protect against short-lived threats. “We thought the ESCRT pathway was an Achilles heel that viruses like HIV and Ebola could always exploit as they bud off and infect new cells,” Elde said. “RetroCHMP3 flipped the script, making the viruses vulnerable. Moving forward, we hope to learn from this lesson and use it to counter viral diseases.”
More specifically, that lesson “raises the possibility that an intervention that slows down the process may be inconsequential for the host, but provide us with a new anti-retroviral,” added Simon.