In military strategy, cutting off an enemy’s supply line is an attrition tactic that armies have used since before the time of Genghis Khan. Scientists have often looked toward this approach when fighting disease, especially for cancer. Now, researchers at the National Institutes of Health (NIH) have found that a small-molecule drug called ONC201, originally shown to induce transcription of the tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) and destroy cancer cells by activating TRAIL death receptors, has a novel mechanism of action in breast cancer cells.
Findings from the new study were published recently in Oncotarget, in an article entitled “ONC201 Kills Breast Cancer Cells In Vitro by Targeting Mitochondria.”
ONC201 was originally identified as a small molecule that inhibits both Akt and ERK (or, extracellular-regulated kinase), resulting in dephosphorylation of Foxo3a and thereby induces TRAIL transcription. TRAIL, a member of the TNF family of ligands, causes caspase-dependent apoptosis through activation of its receptors, death receptor 4 and 5 (DR4 and DR5, respectively).
Recently, investigators from Fox Chase Cancer Center and MD Anderson Cancer Center reported that ONC201 induces cell death via cell stress mechanisms, independent of TRAIL transcription. Gene expression profiling analysis revealed that ONC201 induces endoplasmic reticulum (ER) stress or integrated stress response-related genes, such as activating transcription factor 4 (ATF4) and C/EBP-homologous protein (CHOP). These findings led investigators to down the path toward the current study.
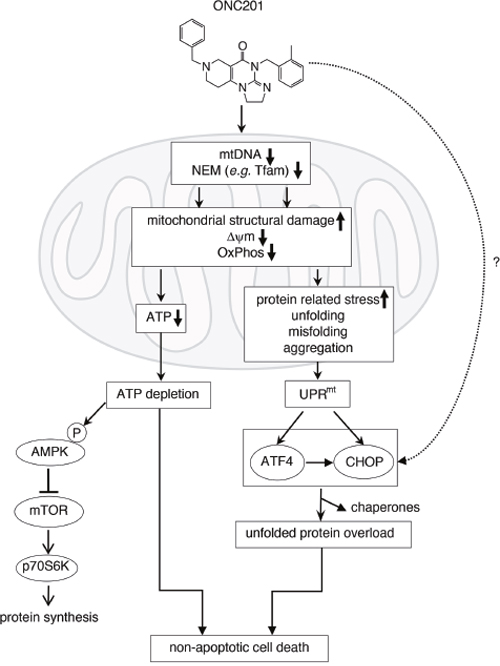
“…we examined ONC201 toxicity on multiple human breast and endometrial cancer cell lines. ONC201 attenuated cell viability in all cancer cell lines tested,” the authors wrote. “Unexpectedly, ONC201 toxicity was not dependent on either TRAIL receptors nor caspases. Time-lapse live cell imaging revealed that ONC201 induces cell membrane ballooning followed by rupture, distinct from the morphology of cells undergoing apoptosis. Further investigation found that ONC201 induces phosphorylation of AMP [adenosine monophosphate]-dependent kinase and ATP [adenosine triphosphate] loss. Cytotoxicity and ATP depletion were significantly enhanced in the absence of glucose, suggesting that ONC201 targets mitochondrial respiration.”
The research team found that ONC201 inhibits mitochondrial respiration and induces mitochondrial structural damage. Moreover, they found ONC201 reduces mitochondrial DNA copy number. Importantly, cells dependent on glycolysis, such as fumarate hydratase-deficient cancer cells, and multiple cancer cell lines with reduced amounts of mitochondrial DNA were resistant to ONC201. ONC201 induced ATF4 and CHOP in breast cancer cells, and the stress response was partially dependent on the mitochondrial effects of ONC201.
“RNAseq analysis revealed that ONC201 suppresses the expression of multiple mtDNA [mitochondrial DNA)-encoded genes and nuclear-encoded mitochondrial genes involved in oxidative phosphorylation and other mitochondrial functions,” the authors penned. “Our data demonstrate that ONC201 kills cancer cells by disrupting mitochondrial function and further suggests that cancer cells that are dependent on glycolysis will be resistant to ONC201.”
Senior study investigator Stanley Lipkowitz, M.D., Ph.D., chief of the Women's Malignancies Branch at the National Cancer Institute (NCI), concluded that “our work identifies a novel mechanism of ONC201 cytotoxicity that is based on the disruption of mitochondrial function, leading to ATP depletion and cell death in cancer cells that are dependent on mitochondrial respiration. Our study also suggests that cancer cells that are dependent on glycolysis will be resistant to ONC201.”