Stem cell biology, tissue bioengineering, and immunology are a few fields that are forging novel therapeutic directions in treating diabetes mellitus, an increasingly important source of global morbidity and mortality. Examples of advances that stem from cross-disciplinary efforts include reprogramming stem cells into pancreatic islet cells, engineering a micro-pancreas, creating hypoimmunogenic stem cells, and encapsulating genetically modified cells that sense blood sugar levels. Concomitantly, we continue to learn about the genetics and genomics of diabetes, with hopes to better characterize the relevant chromosomal variants, quantitate their relative contributions, and develop more informative animal models.
Interrogating the genetics and genomics of diabetes mellitus
“About 600 different genomic locations that are associated with the risk of diabetes and disease progression have already been identified,” says Michael L. Stitzel, PhD, associate professor at the Jackson Laboratory. The various genes and pathways that were implicated in the pathogenesis of diabetes converge on a shared output, which is uncontrolled blood sugar that results from pancreatic islet dysfunction.
“We have used a high-throughput screen to understand how different genetic variants alter transcriptional regulatory programs that control pancreatic islet cell identity and function,” Stitzel reports. For the first time, Stitzel and colleagues interrogated thousands of single nucleotide polymorphisms to understand the genetic risk of type 2 diabetes mellitus and identified several variants that mediate the transcriptional response to endoplasmic reticulum stress.
“I fully expect that we will also find less penetrant single nucleotide polymorphisms that are important,” Stitzel adds. “All these variants will give us another [way to understand] the genetic architecture of diabetes.”
In diabetes, as in many other conditions, the study of genetic variants often requires animal models—models that could be improved through an understanding of evolutionary history. Mouse and human lineages diverged about 75 million years ago, and genetic variants in mice and those in humans emerged separately. To better understand the cross-species relevance of genetic variants associated with diabetes mellitus, Stitzel and colleagues examined the gene arrangement and the overall sequence conservation of the human chromosome 15 locus linked to type 2 diabetes.
Functional genomics and genetic fine mapping of this locus identified a high-risk allele that provides a transcription factor binding site and could function as a super-enhancer. “Functional mapping and targeted crosses,” Stitzel points out, “will help build better and more precise animal models that can recapitulate aspects of the disease and be more useful for preclinical studies.”
Cellular differentiation is intimately shaped by the environment
“For over a decade, we have been trying to convert stem cells into the insulin-secreting pancreatic β cells that are lost in patients with diabetes,” says Jeffrey R. Millman, PhD, associate professor of medicine and biomedical engineering at the Washington University School of Medicine. Several years ago, Millman and colleagues described, for the first time, the in vitro process of generating functional pancreatic β cells from stem cells.
“Before that, the field could only make various types of progenitor cells that secreted insulin, but they did not seem to be true β cells,” Millman notes. Work in Millman’s laboratory includes the characterization of factors that govern β cell differentiation, and the development of manufacturing processes that can generate insulin-secreting cells more robustly and at a higher scale.
A puzzling finding from Millman’s initial work was that differentiation didn’t occur unless the cells were in aggregates of about 2,000. “If we tried to repeat the protocol with cells that were floating around, the process did not work,” Millman recalls. “This meant we did not understand something about the process.”
Subsequent experiments by Millman and colleagues identified the rigidity of the surrounding microenvironment as a critical factor. “One thing that we and others did not seem to initially account for is that cells are squishy,” Millman remarks. Unlike cells, Petri dishes are very rigid.
In a critical experiment, Millman and colleagues observed a linear relationship between the ability of cells to differentiate toward endocrine directions and the rigidity of the microenvironment they grew on. To circumvent the need for expensive manufacturing and scaling, scientists in Millman’s laboratory developed a strategy that did not require specialized biomaterials. “We designed a molecular screen that used small molecules to target various known cellular consequences of the surrounding microenvironment as a result of differences in rigidity,” Millman details.
This approach identified latrunculin A, an actin cytoskeleton depolymerizing compound, as a small molecule that prevents the endocrine induction of human pluripotent stem cells. The attachment of cells to rigid surfaces leads to actin polymerization, whereas on soft surfaces, actin polymerizes to a lesser degree. “By using latrunculin, we were able to ‘blind’ the cells,” Millman explains. “They could no longer tell if they were on a rigid or on a soft surface.”
The decrease in polymerized actin had several downstream consequences in the cells, including epigenetic, signaling pathway, and transcriptional changes that modulate gene expression. These findings informed the development of a robust and rigorous protocol to differentiate stem cells into islet cells of the endocrine pancreas.
“There are additional cell types like cardiac muscles that we can manufacture at scale in the laboratory and that we can make more useful for therapy,” Millman points out. “Once all the technical issues are worked out, there will be a lot more manufacturing of cells and tissues.”
The most critical obstacle for using stem cell–derived products therapeutically is the danger of immune rejection. “The field does not have a conclusive solution to this challenge yet,” Millman notes. Immunosuppressive medication is one option, but it is not desirable, particularly when the condition that requires reprogrammed cells is autoimmune in nature.
“I think the answer is using CRISPR to genetically engineer the cells so that they are tolerated by the immune system,” Millman suggests. “This is an example of how stem cell technology and genetic engineering can together help manufacture cell types that can be used for therapy.”
The engineered micro-pancreas
“Our work is based on research that Professor Eduardo Mitrani conducted at the Hebrew University of Jerusalem,” says Nikolai Kunicher, PhD, CEO of Betalin Therapeutics. “We hope to soon progress toward Phase I trials in the United States and the United Kingdom.” Current preclinical studies at Betalin are based on the development of a decellularized porcine lung–derived microscaffold, which provides the extracellular matrix to host human pancreatic β cells and mimic their natural environment.
By combining human islets with a micro-organ matrix, Betalin creates what it calls the Engineered Micro Pancreas (EMP). “Once we will implant the EMP under a patient’s skin, the cells will secrete insulin into their bloodstream,” Kunicher explains. In a mouse model of disease, Betalin’s EMP reversed hyperglycemia and maintained normal glycemic levels for up to three months, as compared with approximately one week in the case of naked β cells.
The main obstacle in the clinical translation of the EMP at a large scale is that cells are currently obtained from donors. “That places a limit on the number of patients that could be treated using this approach,” Kunicher notes. However, this difficulty can be circumvented by relying on technologies that reprogram a patient’s own cells into induced pluripotent stem cells. “The use of autologous cells,” Kunicher adds, “will also eliminate the need for immunosuppressive drugs.”
Creating hypoimmunogenic stem cells
“We are moving into an era where human stem cell therapy is hopefully becoming another tool in our arsenal to help patients,” says Matthias Hebrok, PhD, Hurlbut-Johnson Distinguished Professor in Diabetes Research at the University of California, San Francisco. In a recent study, Hebrok and colleagues used gene editing to selectively delete from the surface of human pluripotent stem cells molecules that are being recognized by the immune system.
This strategy promises to generate cells that would be tolerated by a stem cell transplant recipient. “Other scientists have performed versions of this before, but what sets us apart,” Hebrok asserts, “is that we have maintained specific molecules being recognized by our immune system.”
In this proof-of-principle approach, Hebrok and colleagues retained the common HLA class I allele HLA-A2, which is shared by a large percentage of the human population, increasing the likelihood of compatibility with potential transplant recipients. HLA-E, a noncanonical MHC molecule that recognizes natural killer cells and retains immune surveillance, was also retained.
The hypoimmunogenic human pluripotent stem cells generated by this technology are ideally positioned to escape rejection by the immune system. “The smart modulation of molecules that the immune system recognizes might help at a minimum reduce the level of immunosuppressive drugs and make stem cell therapies become a reality,” Hebrok proposes.
Selectively deleting molecules that would increase the risk of graft rejection may be helpful in areas other than the treatment of diabetes. For example, it also opens avenues toward generating many different cell types that are damaged by disease, such as cardiomyocytes or dopaminergic neurons. “In the long run,” Hebrok predicts, “we will be able to either make the immune system recognize these engineered cells as their own or at least not be bothered by them.”
A pinhead-sized cell-in-a-box platform
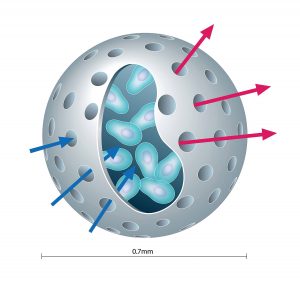
“What distinguishes our technology is the protective, bioinert capsule that we use to encapsulate genetically modified live cells to treat diseases,” says Kenneth L. Waggoner, the CEO of PharmaCyte Biotech. In PharmaCyte’s Cell-in-a-Box technology, 0.7–0.8-mm spherical capsules made of cellulose (a substance that is bioinert in the human body) house genetically engineered live cells. The semipermeable capsule allows the exchange of nutrients and waste products between the encapsulated cells and the blood but prevents the entry of immune cells or the escape of the capsule’s cells.
The bioinert nature of the capsule prevents its breakdown over time. In many other encapsulation technologies, breakdowns occur that risk eliciting an immune response against the encapsulated material. PharmaCyte asserts that its capsules do not degrade even after residing in the human body for two years, and that they do not cause tissue inflammation or damage. The company designs its encapsulated cells to maintain their function even after they have been cryopreserved for over five years.
“One of our immediate goals is to validate this technology in the clinic,” Waggoner states. “We will do that in a clinical trial for pancreatic cancer.” PharmaCyte’s scientists are currently developing the Cell-in-a-Box technology into therapeutic interventions for pancreatic and other difficult to treat cancers, malignant ascites, and diabetes.
Each capsule contains approximately 10,000–20,000 live cells. (The number varies depending on the application.) The capsules for PharmaCyte’s pancreatic cancer product are implanted in the blood supply as close to the tumor as possible. “The encapsulated cells act as an artificial liver to convert an inactive chemotherapy prodrug, which is administered intravenously, into the active form,” Waggoner explains.
The diabetes program at PharmaCyte encapsulates Melligen cells and genetically modified stem cells. The Melligen cells, developed by the University of Technology Sydney, are engineered human liver cells that secrete and release insulin in response to blood glucose concentrations. In immunosuppressed diabetic mice, the Melligen cells normalized blood glucose levels, and their ability to secrete insulin was not affected by the presence of inflammatory cytokines.
“We are working with the University of Technology Sydney to improve the Melligen cells,” says Waggoner. The goal is to ensure that the cells remain stable and continue to enhance insulin production after the required number of passages.

