Sponsored content brought to you by
Antimicrobial resistance is one of the top ten threats to public health worldwide. It occurs when bacteria, viruses, fungi, and parasites do not respond to antimicrobial drugs anymore. Drug-resistant microbes readily arise from DNA replication errors. Resistant microbes then grow and expand quickly, e.g., through frequent bacterial replication. The resistant genes can mobilize and transfer themselves to different genomes and species at the same time.
Consequently, the use of traditional medicines becomes ineffective. As treatment options are gradually decimated by these resistances, there is a risk that infections will spread more quickly and lead to severe illness and even death—similar to the situation before the discovery of antimicrobial medicine in the early 20th century. One of the greatest threats is currently posed by resistant bacteria—referred to as antibiotic resistance.
New hope: bacteriophages
One attractive alternative to traditional antibiotics is phage therapy. Bacteriophages are viruses with antibacterial properties. They specifically infect bacteria (causing bacterial cell lysis) without any adverse effects to humans or animals. Furthermore, bacteriophages can attack specific types of bacteria in contrast to conventional antibiotics, which often cover a broad spectrum of bacteria. The therapy also appears to be less susceptible to resistance.
Crystal violet staining reveals inhibitory effects on biofilms
Biofilms are colonies of microorganisms that form a protective layer of extracellular polymeric substances. As biofilms are commonly more resistant to antimicrobials, research is focused on combating and containing them.
An easy method to visualize and quantify biofilms is to stain and analyze them with an absorbance-based microplate reader. In the following example, a 1 McFarland unit of the bacterium E. coli strand XL10 was diluted 1:15 and filled in a 96-well plate in LB Broth with 150 µL per well. After 48 h of incubation at 37°C the novel bacteriophage (APTC-EC-2A) was added in 200 µL of 5 x 108 or 5 x 109 plaque forming units per mL (Figure 1). The E. coli and bacteriophages were further incubated for 24 h.
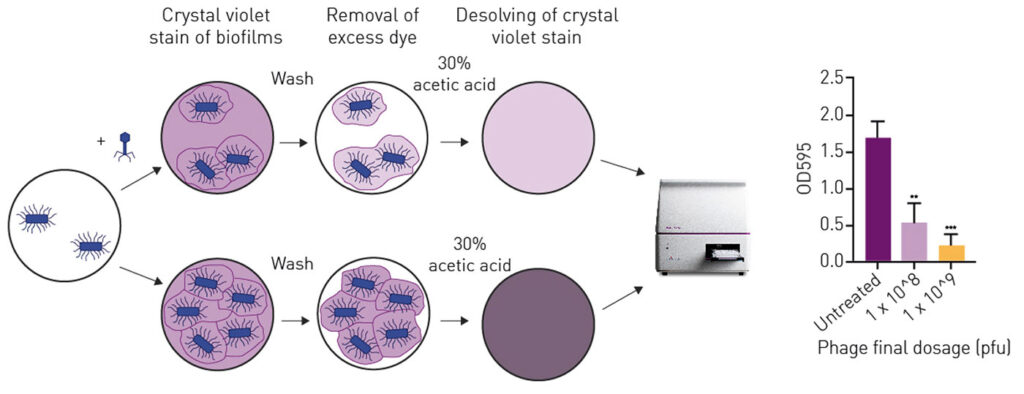
In order to visualise the possible effect of the bacteriophages on the biofilm, the wells were subsequently stained with crystal violet which binds to proteins and DNA. Bacteria that undergo cell death lose their adherence within the biofilm and are washed away before the staining process, reducing the amount of crystal violet staining in a culture. 200 µL 0.1% crystal violet were applied per well and incubated for 15 min. After washing away unbound crystal violet, bound dye was dissolved in 30 % acetic acid and absorbance was read at 595 nm on a BMG LABTECH microplate reader. Untreated E. coli were used as positive control and pure LB broth was used as negative control.
As displayed in the right panel of Figure 1, the treatment of E. coli led to a dose-dependent decrease in biofilm biomass. The bacteriophage APTC-EC-1A significantly reduced the biomass of E. coli XL Gold up to 70 %.
The assay can be read with almost any absorbance-based microplate reader, making the method accessible to many research groups. As research to combat antibiotic resistance also involves the investigation of a large number of possible candidates, the method has great potential for the discovery of new alternatives to conventional antibiotics.
The microplate readers from BMG LABTECH are ideally suited for analyzing biofilms using the crystal violet method. Thanks to their ultra-fast CCD spectrometer, they can also be used for many other absorbance measurements in the 220-1000 nm range. The incubation function and the long-term heavy-duty shaking option support kinetic growth curves of bacteria or yeasts. Methods based on other detection modes, such as BacTiter-Glo™, are of course also covered.
 If you are interested in further details about microplate-based evaluation of microbial assays, please contact us at: [email protected].
If you are interested in further details about microplate-based evaluation of microbial assays, please contact us at: [email protected].




