Sponsored content brought to you by


Vaccines have been developed at record-breaking speeds to fight the COVID-19 pandemic and are already saving lives. At the time of writing, over 2.55 billion COVID-19 vaccine doses have been administered globally and are paving the way to a return to a “new normal.”
However, there is still a pressing need to develop more vaccine options to further increase global access and prepare for future infectious disease challenges. To aid in the manufacturing of new vaccines, Pichia pastoris yeast, a promising protein expression system, could be the perfect solution.

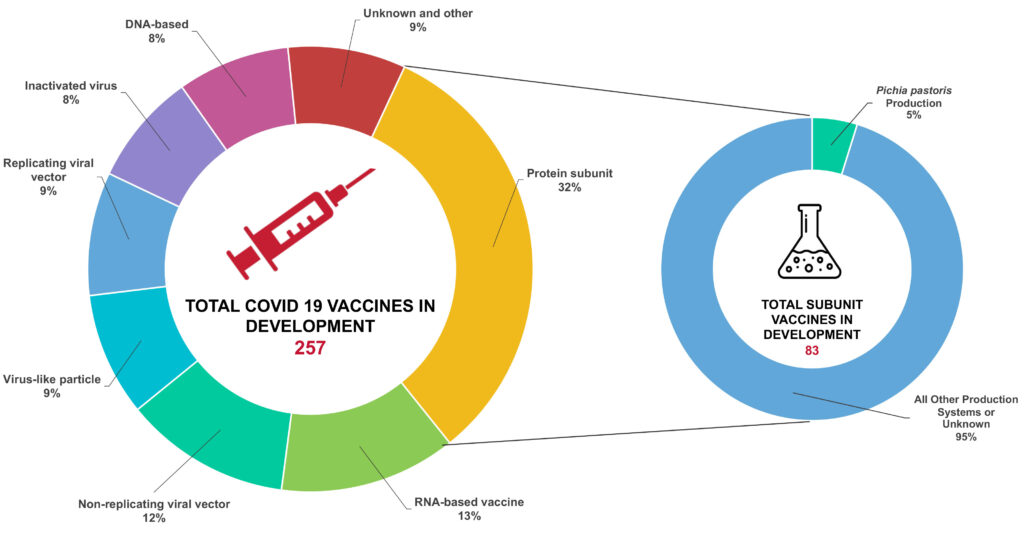
For targeted and safe protection against COVID-19, protein subunit vaccines are the predominant vaccine type in development against SARS-COV-2. These are vaccines containing a fragment of a pathogen that is able to trigger the immune response to build protection against a disease. For a vaccine against SARS-COV-2, the receptor binding domain (RBD), part of the spike protein, is the preferred fragment to express. Subunit vaccines are considered safe since they don’t contain the pathogen itself and additionally minimize the risk of side effects compared to several other vaccine types.
However, challenges still exist in the development of protein subunit vaccines. Compared to older vaccine types, such as inactivated virus or live-attenuated virus vaccines, fully recombinant vaccines require more complex manufacturing and downstream processing. We also have already seen the difficulty of ensuring global supply of COVID-19 vaccines, which is essential for reaching an end to the COVID-19 pandemic. To deliver global solutions, vaccines need to be produced at scale and priced affordably.
To address these challenges, Pichia pastoris has many features that make it attractive for protein subunit vaccine production. Notably, Pichia can grow to ultra-high cell densities, an important factor for efficient and low-cost protein production. As a result, it’s suitable for large-scale fermentation in bioreactors and has been shown to produce higher amounts of protein than other commonly used eukaryotic expression systems such as Chinese hamster ovary (CHO) cells, Saccharomyces cerevisiae, and insect cells. Moreover, as a eukaryote, Pichia can also handle more complex proteins than bacterial expression systems, but without the increased cost or complexity of using mammalian cells.
Furthermore, traditional challenges to the use of Pichia pastoris have been overcome with recent innovations. As a methylotrophic yeast, Pichia is conventionally grown on methanol. However, methanol and its metabolites are highly toxic to the growth of Pichia cells and require explosion-proof equipment, which makes scaling up production a costly and onerous task.
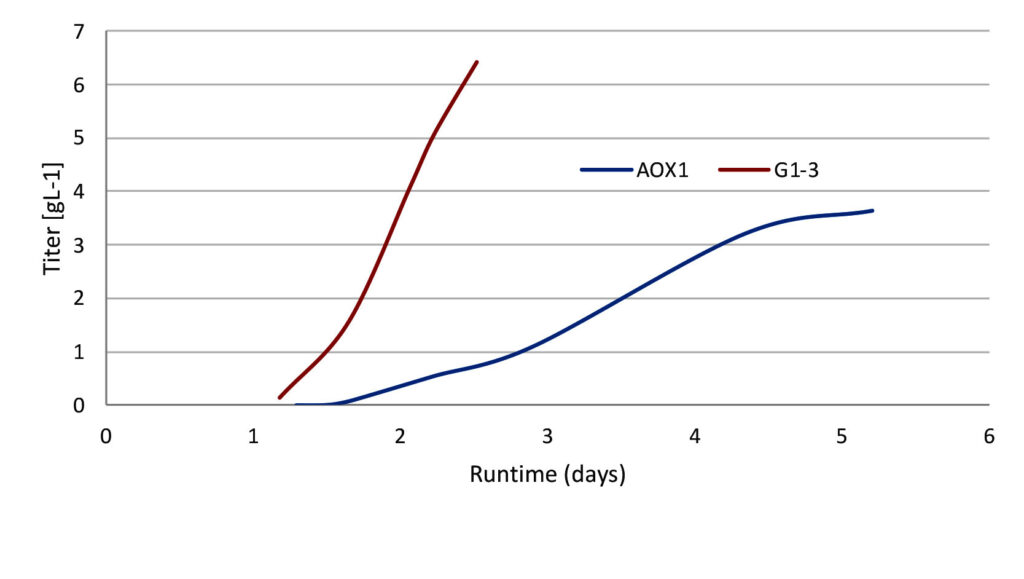
Fortunately, the recent development of glucose-regulated Pichia expression systems, such as Lonza’s XS Pichia, solves the issue by eliminating the need for methanol. It has the additional benefit of increasing productivity by up to 50%, which means Pichia pastoris can now rival the productivity of Escherichia coli, allowing for low-cost and high-quality protein production for vaccines and therapies.
While the idea of using yeast to produce vaccines may seem unusual, yeast production systems have previously been used for several common vaccines. For example, Engerix-B®, Heplisav-B®, and Recombivax HB® have been approved for vaccination against hepatitis B. Gardasil®, an HPV vaccine, is also produced using yeast. Pichia pastoris has over 70 non-vaccine products on the market or in late-stage development and is now being explored as a vaccine production system.
Given the advantages discussed, Pichia pastoris is being used as a protein expression system for manufacturing several COVID-19 vaccine candidates. Subunit vaccines being produced using Pichia pastoris are being investigated by Oxford University, Baylor College of Medicine, MIT/Serum Institute of India, and Cuba’s Center for Genetic Engineering and Biotechnology.
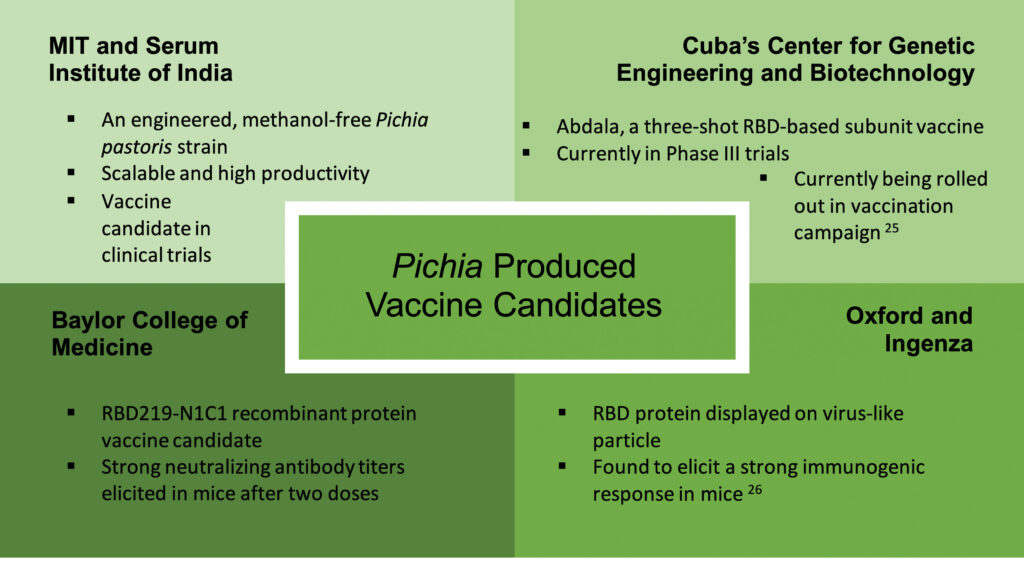
The MIT-engineered Pichia pastoris strain has already been used to produce vaccine supply for clinical trials and has even been scaled to a 1,200-L production facility, showing promise for future commercial manufacturing. However, only a fraction of COVID-19 vaccines in development are relying on Pichia pastoris as an expression system, leaving much potential untapped.
Looking forward, the task of supplying the world with sufficient COVID-19 vaccines remains a significant challenge, but also an opportunity for more firsts in the world of vaccine manufacturing. We have already seen the first approval of an mRNA vaccine in record- breaking time. Perhaps we may also see the first approval of a Pichia pastoris–manufactured vaccine that paves the way for a future of efficient and cost-effective vaccine manufacturing. With Pichia, we may be able to meet the global COVID-19 vaccine demand and reduce the burden of infectious diseases for years to come.
Learn more pharma.lonza.com/xstechnologies
Lisa Tu is a former intern at Lonza, and Devarshi Kapadia is product manager, GS Licensing, at Lonza ([email protected]).


