Researchers at Karolinska Institutet in Sweden have identified an experimental antidiabetic compound that can protect pancreatic ß cells from “overloading” under the metabolic stresses of diabetes, and so prevent high blood glucose and the progression of diabetes in mouse models. The group’s findings demonstrated that the hypoxia-inducible factor-1α (HIF-1α) inhibitor, PX-478—which has already been evaluated in clinical trials against cancer—can interrupt a mechanism that contributes to diabetes and, when tested in human organoids, showed preliminary promise as a therapeutic agent.
“In diabetes, beta cells are challenged to produce high amounts of insulin,” explained Erwin Ilegems, senior researcher at the department of molecular medicine and surgery, Karolinska Institutet. “Our study shows that this leads to a hypoxic state that increases the levels of HIF-1α protein, which in turn reduces beta cell activity. By treating diabetic mice with the HIF-1α inhibitor PX-478 we successfully decreased their blood glucose levels.”
Ilegems is senior author of the team’s published paper in Science Translational Medicine, which is titled “HIF-1α inhibitor PX-478 preserves pancreatic ß cell function in diabetes,” in which they noted, “We show that, under metabolic overload, the HIF-1α inhibitor PX-478 restored normal insulin secretion in response to glucose in mouse islets and increased the stimulation index of glucose-induced insulin secretion in human islet organoids.”
The onset of obesity-linked type 2 diabetes is a consequence of insufficient insulin secretion to compensate for insulin resistance, the authors explained. During the early stages of the disease, expansion of pancreatic ß cell mass and enhanced pancreatic ß cell function can counteract the insulin resistance to maintain normal glucose levels. However, as the disease progresses, sustained “metabolic overload” will lead to ß cell dysfunction.
It is well established that the production of insulin decreases during the progression of diabetes, so therapeutic strategies have so far mostly focused on improving the insulin production of ß cells. However, this approach has not been as successful as initially predicted. Studies have shown that ß cell function may already be down to 50–80% of normal at the time of type 2 diabetes onset, and this impaired ß cell function correlates with impaired control of glucose irrespective of the therapeutic intervention, the team pointed out. And while several families of glucose-lowering agents are currently used in diabetes therapy, none can stop or reverse the progression of the disease. “Therefore, preservation or recovery of ß cell function is an important strategy in the treatment of type 2 diabetes,” the team stated.
“Current therapies targeting beta cells have only a temporary positive effect on insulin secretion,” added study co-author Per-Olof Berggren, PhD, professor at the department of molecular medicine and surgery, Karolinska Institutet. “In the long-term, these drugs lead to beta cell exhaustion.”
Because of the high energy demands of insulin secretion, pancreatic ß cells consume large amounts of oxygen in mitochondrial respiration, the authors continued. “The dependence of ß cell function on oxygen availability has previously been explored in two studies showing that in vitro exposure of ß cells to high glucose concentrations leads to a cellular hypoxic phenotype with activation of HIF-1α.”
HIF-1α regulates the response to hypoxia, and has long been recognized as an important cancer drug target. “ … The HIF-1α inhibitor PX-478 was originally developed for cancer therapy and has been tested in clinical trials in patients with advanced solid tumors,” the investigators pointed out.
In collaboration with the research group of Jorge Ruas, PhD, at the department of physiology and pharmacology at Karolinska Institutet, the team has now demonstrated that the antidiabetic effect of PX-478 is mainly due to improved pancreatic ß cell activity.
The investigators tested PX-478 therapy in two mouse models of diabetes. “The db/db mouse is an obesity-linked diabetic model presenting severe insulin resistance that leads to high metabolic overload and subsequent ß cell exhaustion and failure,” they explained. “The STZ-induced diabetic mouse is a lean model of reduced functional ß cell mass in which ß cells are under high metabolic workload.
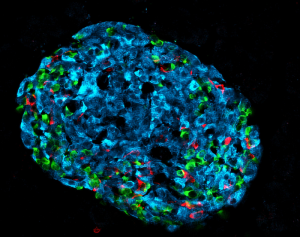
Positive results were also obtained through tests in human organoids. “In response to PX-478 treatment, human islet organoids chronically exposed to high glucose presented improved stimulation index of glucose-induced insulin secretion.”
Unlike other types of treatment, PX-478 ameliorates ß cell activity without amplifying insulin secretion, the investigators pointed out. They believe that this approach can prevent the exhaustion of ß cells and thus be more efficient in the long-term treatment of diabetes. The results of their reported studies, the investigators stated, suggest that “… PX-478 can serve as a promising glucose-lowering therapeutic strategy to preserve ß cell function in diabetes.”
“We are now planning to further investigate the translatability of our findings to hopefully pave the way for future clinical trials,” said Teresa Pereira, PhD, researcher at the department of medical cell biology, Uppsala University (formerly employed by Karolinska Institutet), who is co-last author of the study. “We will start by investigating the impact of PX-478 on human pancreatic ß cell activity by using ‘humanized’ diabetic mice.”

![Dual-mechanism oral small molecule MBX-2982 acts as GPR119 agonist. [© Celso Pupo - Fotolia.com] Dual-mechanism oral small molecule MBX-2982 acts as GPR119 agonist. [© Celso Pupo - Fotolia.com]](https://www.genengnews.com/wp-content/uploads/2018/08/June25_2010_2776985_DiabetesParaphernalia_SanofiAventisMetabolexDiabetesCollab_Crop2031951925-2.jpg)
