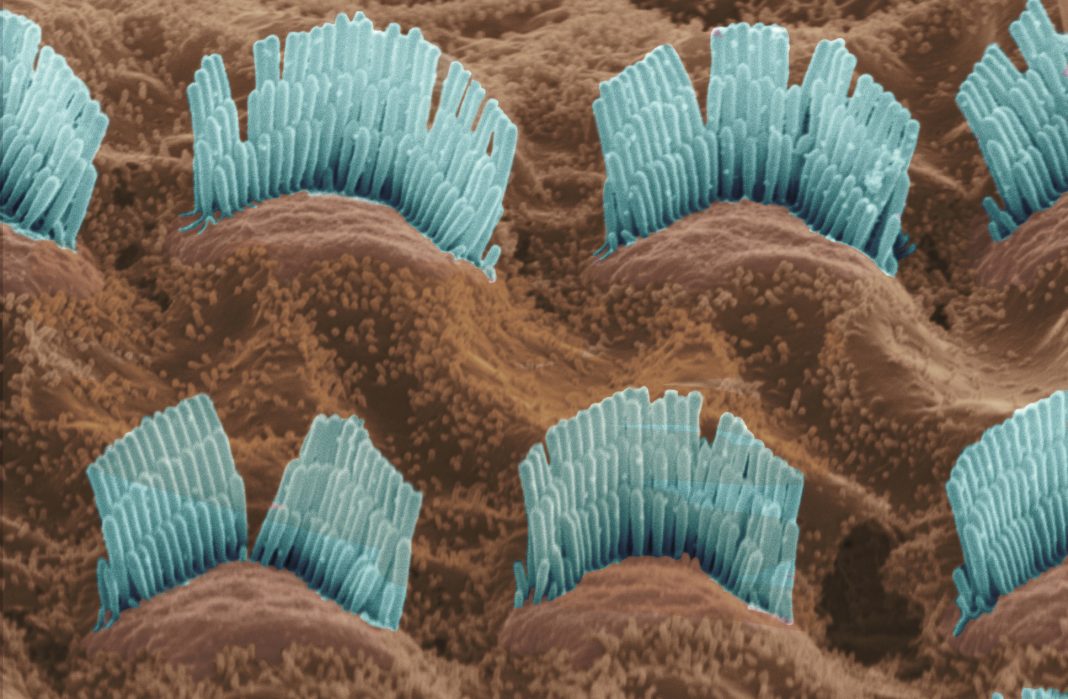
Researchers have identified potential therapeutic targets to prevent hearing loss caused by antibiotics. The findings are particular to aminoglycosides (AGs). Although AGs are capable of treating a wide variety of life-threatening infections, they are also ototoxic. That is, they cause irreversible damage to cochlear hair cells.
To prevent such damage, researchers at Indiana University School of Medicine decided to investigate how AG exposure affects the molecular pathways critical for hair cell function and survival. Ultimately, the researchers zeroed in on a mechanism involving a protein called RIPOR2, or RHO family interacting cell polarization regulator 2. Mutations in this protein have been associated with hearing loss.
In the current study, RIPOR2 mutations were not at issue. Instead, RIPOR2 translocations were detected.
The implications of this finding were discussed in a paper “RIPOR2-mediated autophagy dysfunction is critical for aminoglycoside-induced hearing loss” that appeared in the journal Developmental Cell. According to this article, “translocation of RIPOR2 in murine hair cells from stereocilia to the pericuticular area” is triggered within minutes by gentamicin, a representative AG antibiotic. Subsequently, RIPOR2 interacts with a central autophagy component, GABARAP. The result: autophagy—or rather, autophagy dysfunction.
“Reducing the expression of RIPOR2 or GABARAP completely prevents AG-induced hair cell death and subsequent hearing loss in mice,” the article’s authors wrote. “Additionally, abolishing the expression of PINK1 or Parkin, two key mitochondrial autophagy proteins, prevents hair cell death and subsequent hearing loss caused by AG.”
The researchers, led by Indiana University’s Bo Zhao, PhD, developed one of the first laboratory models that’s insusceptible to aminoglycoside-induced hearing loss. “Our work,” he explained, “identifies multiple potential therapeutic targets for preventing hearing loss caused by aminoglycosides.”
Zhao is assistant professor of otolaryngology—head and neck surgery, and he heads a laboratory that investigates the molecular mechanisms underlying hearing loss. In the current study, his laboratory used biochemical screening to identify proteins found in hair cells. They first discovered that aminoglycosides bound to the protein RIPOR2, which is required for auditory perception.
“As aminoglycosides specifically trigger a rapid localization change of RIPOR2 in hair cells, we hypothesize that RIPOR2 is essential for aminoglycoside-induced hair cell death,” Zhao said.
Aminoglycosides for nearly a century have been used to treat severe infections. Although the drug is a first-line treatment for life-threatening infections—particularly in developing countries—due to their low cost and low incidence of antibiotic resistance, it has been reported to cause hair cell death and subsequent permanent hearing loss among 20–47% of patients.
Ototoxicity—hearing loss caused by medication—is one of the main causes of hearing loss in humans. More than 48 million people in the United States experience trouble hearing.
To clarify the mechanisms underlying aminoglycoside-related ototoxicity, the researchers developed a model in the lab that has normal hearing but significantly decreased RIPOR2 expression. Through these experiments, Zhao said the model had neither significant hair cell death nor hearing loss after treatment of aminoglycosides.
“We then discovered RIPOR2 regulates the autophagy pathway in hair cells,” said Jinan Li, PhD, postdoctoral fellow in the Zhao lab and first author of the paper. “Knowing this, we developed other laboratory models without the expression of several key autophagy proteins that did not exhibit hair cell death or hearing loss when treated with the antibiotic.”
Zhao, Li, and the other authors of the current study say that the proteins they identified could potentially be used as drug targets to prevent aminoglycoside-induced hearing loss.