Using a special X-ray microscopy technique, a group of physicists, pathologists, and lung specialists were able to image changes caused by SARS-CoV-2 in the structure of alveoli (the tiny air sacs in the lung) and the vasculature of lung tissue. This three-dimensional (3D) imaging technique—based on multi-scale phase contrast X-ray tomography—offers high resolution and three-dimensional representation of damaged lung tissue following severe COVID-19. In this study, it was used to investigate the parenchymal architecture of unstained damaged lung tissue from patients who succumbed to COVID-19.
The results of the study were published in eLife in a paper titled, “3D virtual pathohistology of lung tissue from COVID-19 patients based on phase contrast x-ray tomography.”
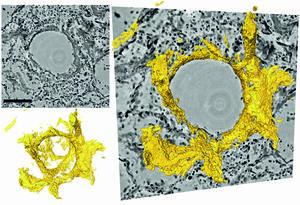
In severe COVID-19 disease, the researchers observed significant changes in the vasculature, inflammation, blood clots, and “hyaline membranes,” which are composed of proteins and dead cells deposited on the alveolar walls, which make gas exchange difficult or impossible.
With their new imaging approach, these changes can be visualized for the first time in larger tissue volumes, without cutting and staining or damaging the tissue as in conventional histology. It is particularly well suited for tracing small blood vessels and their branches in three dimensions, localizing cells of the immune systems which are recruited to the inflammation sites, and measuring the thickness of the alveolar walls. Due to the three-dimensional reconstruction, the data could also be used to simulate gas exchange.
“Using zoom tomography, large areas of lung tissue embedded in wax can be scanned enabling detailed examination to locate particularly interesting areas around inflammation, blood vessels, or bronchial tubes,” said lead author Tim Salditt, PhD, professor at the Institute of X-ray Physics at the University of Göttingen. Since X-rays penetrate deep into tissue, this enables scientists to understand the relation between the microscopic tissue structure and the larger functional architecture of an organ. This is important, for example, to visualize the tree of blood vessels down to the smallest capillaries.
Based on this first proof-of-concept study, the authors propose multi-scale phase contrast X-ray tomography as a tool “to unravel the pathophysiology of COVID-19, extending conventional histology by a third dimension and allowing for full quantification of tissue remodeling.”
The authors foresee that this new X-ray technique will be an extension to traditional histology and histopathology, areas of study which go back to the 19th century when optical microscopes had just become available and pathologists could thereby unravel the microscopic origins of many diseases. Even today, pathologists still follow the same basic steps to prepare and investigate tissue: chemical fixation, slicing, staining, and microscopy. This traditional approach, however, is not sufficient if three-dimensional images are required or if large volumes have to be screened, digitalized, or analyzed with computer programs.
Three-dimensional imaging is well known from medical computerized tomography (CT). However, the resolution and contrast of this conventional technique are not sufficient to detect the tissue structure with cellular or sub-cellular resolution. Therefore, the authors used “phase contrast,” which exploits the different propagation velocities of X-rays in tissue to generate an intensity pattern on the detector. Salditt and his research group at the Institute for X-ray Physics developed special illumination optics and algorithms to reconstruct sharp images from these patterns, an approach which they have now adapted for the study of lung tissue affected by severe progression of COVID-19. The Göttingen team could record lung tissue at scalable size and resolution, yielding both larger overviews and close-up reconstructions. Depending on the setting, their method can even yield structural details below the resolution of conventional light microscopy. To achieve this, the researchers used highly powerful X-ray radiation generated at the PETRAIII storage ring of the German Electron Synchrotron (DESY) in Hamburg.
By combining parallel and cone beam geometry, the authors wrote, autopsy samples with a maximum cross section of 4 mm are scanned and reconstructed at a resolution and image quality which allows for the segmentation of individual cells.
To offer more detail, the authors wrote that, “using the zoom capability of the cone beam geometry, regions-of-interest are reconstructed with a minimum voxel size of 167 nm.” They performed 3D visualization of the diffuse alveolar damage with its prominent hyaline membrane formation, by mapping the 3D distribution and density of lymphocytes infiltrating the tissue, and by providing histograms of characteristic distances from tissue interior to the closest air compartment.
As was the case when the modern microscope was invented 150 years ago, significant progress has resulted from collaboration between physicists and medical researchers. The interdisciplinary research team hopes that the new method will support the development of treatment methods, medicines to prevent or alleviate severe lung damage in COVID-19, or to promote regeneration and recovery. “It is only when we can clearly see and understand what is really going on, that we can develop targeted interventions and drugs,” added Danny Jonigk, at Medical University Hannover, who led the medical part of the interdisciplinary study.