The Melanoma Antigen Gene (MAGE) family consists of more than 40 proteins in humans, most of which are only present in the testes under healthy conditions. However, in many cancers, these proteins are found in high levels in tissues where they are not usually expressed and are believed to play a role in promoting cancer progression. Researchers from the Bhogaraju group at the European Molecular Biology Laboratory (EMBL) Grenoble have gained new insights into how these proteins bind their targets. The findings could potentially aid in the development of drugs against chemotherapy- or radiotherapy-resistant cancers.
The findings are published in The EMBO Journal, in an article titled, “Structural basis for RAD18 regulation by MAGEA4 and its implications for RING ubiquitin ligase binding by MAGE family proteins.”
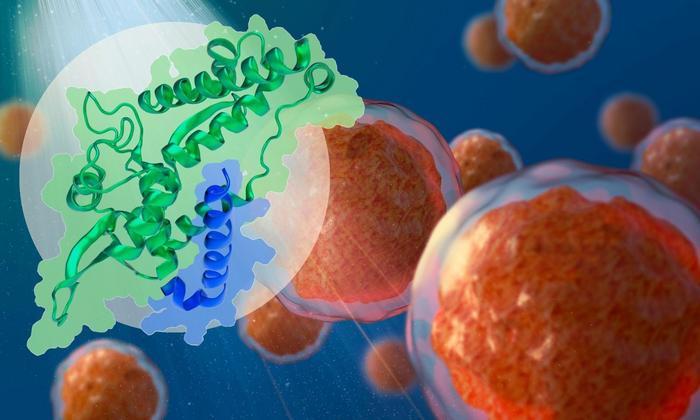
“MAGEA4 is a cancer-testis antigen primarily expressed in the testes but aberrantly overexpressed in several cancers,” the researchers wrote. “MAGEA4 interacts with the RING ubiquitin ligase RAD18 and activates translesion DNA synthesis (TLS), potentially favoring tumor evolution. Here, we employed NMR and AlphaFold2 (AF) to elucidate the interaction mode between RAD18 and MAGEA4, and reveal that the RAD6-binding domain (R6BD) of RAD18 occupies a groove in the C-terminal winged-helix subdomain of MAGEA4.”
The Bhogaraju group at EMBL Grenoble uses structural and cell biology-based approaches to study such ubiquitin-based pathways in normal physiology and disease. The team, in collaboration with the Hennig group at EMBL Heidelberg, looked more deeply into the interaction between the proteins MAGEA4 and RAD18, using AlphaFold, an artificial intelligence–based tool that allows scientists to predict the structure of proteins.
The team found that MAGEA4 has a groove that can bind a section of the RAD18 protein, which prevents the latter from attaching ubiquitin groups to itself and subsequently getting degraded.
The researchers also found a very similar groove in another MAGE family protein, which is used to regulate a different cancer-promoting protein. They believe this groove may be a general feature of the MAGE family, used to mediate binding to cancer-relevant proteins.
In addition to the groove, the scientists also discovered that two parts within the RAD18 protein interact with each other, which helps it attach the ubiquitin tag to a protein which promotes cancer cell survival.
“We are excited with these data because our findings seem to be applicable to many MAGEs opening a gateway into targeting MAGEs which drive cancers,” said Sagar Bhogaraju, PhD, group leader at EMBL Grenoble. “We are currently working on developing methods to screen compounds that bind the newly discovered hotspot of MAGEs.”