Neuroblastoma is the most common childhood tumor occurring outside the brain. Until now, studying genetic changes and their role in neuroblastoma initiation has been challenging due to the lack of suitable laboratory methods. A new model developed by researchers at the University of Sheffield, in collaboration with the St. Anna Children’s Cancer Research Institute in Vienna, replicates the emergence of early neuroblastoma cancer-like cells, providing insight into the genetic pathway of the disease.
Their findings are published in Nature Communications in an article titled, “A human neural crest model reveals the developmental impact of neuroblastoma-associated chromosomal aberrations.”
“Early childhood tumors arise from transformed embryonic cells, which often carry large copy number alterations (CNA),” the researchers wrote. “However, it remains unclear how CNAs contribute to embryonic tumorigenesis due to a lack of suitable models. Here we employ female human embryonic stem cell (hESC) differentiation and single-cell transcriptome and epigenome analysis to assess the effects of chromosome 17q/1q gains, which are prevalent in the embryonal tumor neuroblastoma (NB).”
The international research team found that specific mutations in chromosomes 17 and 1, combined with overactivation of the MYCN gene, play a pivotal role in the development of aggressive neuroblastoma tumors.
Childhood cancer is often diagnosed and detected late, leaving researchers with very little idea of the conditions that led to tumor initiation. Models that recreate the conditions that lead to the appearance of a tumor is needed in order to understand tumor initiation.
The formation of neuroblastoma usually starts in the womb when a group of normal embryonic cells called “trunk neural crest (NC)” become mutated and cancerous.
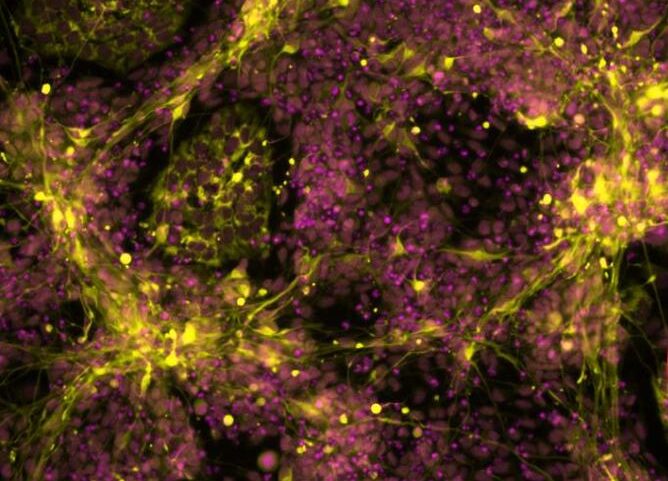
In an interdisciplinary effort spearheaded by stem cell expert Ingrid Saldana, PhD, from the University of Sheffield’s School of Biosciences and computational biologist Luis Montano, PhD, from the St. Anna Children’s Cancer Research Institute in Vienna, the new study found a way in which to use human stem cells to grow trunk NC cells in a petri dish.
These cells carried genetic changes often seen in aggressive neuroblastoma tumours. Using genomics analysis and advanced imaging techniques, the researchers found that the altered cells started behaving like cancer cells and looked very similar to the neuroblastoma cells found in sick children.
The findings offer new hope for the creation of tailored treatments that specifically target the cancer while minimizing the adverse effects experienced by patients from existing therapies.
Anestis Tsakiridis, PhD, from the University of Sheffield’s School of Biosciences and lead author of the study, said: “Our stem cell-based model mimics the early stages of aggressive neuroblastoma formation, providing invaluable insights into the genetic drivers of this devastating childhood cancer. By recreating the conditions that lead to tumor initiation, we will be able to understand better the mechanisms underpinning this process and thus design improved treatment strategies in the longer term.
“This is very important as survival rates for children with aggressive neuroblastoma are poor and most survivors suffer from side effects linked to the harsh treatments currently used, which include potential hearing, fertility, and lung problems.”
Florian Halbritter, PhD, from St. Anna Children’s Cancer Research Institute and second lead author of the study, added: “This was an impressive team effort, breaching geographic and disciplinary boundaries to enable new discoveries in childhood cancer research.”