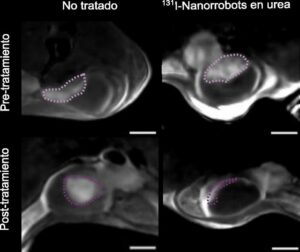
Researchers led by teams at the Institute for Bioengineering of Catalonia (IBEC) and CIC biomaGUNE demonstrated how a single dose of urea-powered, radionuclide-carrying nanorobots successfully reduced the size of bladder tumors in mice by 90%.
![Accumulation of nanorobots in the tumor visualized by microscopy. [IRB Barcelona]](https://www.genengnews.com/wp-content/uploads/2024/01/low-res-10-256x300.jpeg)
The researchers, in collaboration with scientists at the Institute for Research in Biomedicine (IRB Barcelona) and the Autonomous University of Barcelona (UAB), reported on their murine tests with the radio-iodinated nanobots, in Nature Nanotechnology, in a paper titled “Urease-powered nanobots for radionuclide bladder cancer therapy.” In their report the team concluded “These results take us one step closer toward translational applications and highlight the underexplored potential of nanobots for innovative bladder cancer treatment options.”
Bladder cancer has one of the highest incidence rates in the world and ranks as the fourth most common tumour in men. While current treatments involving direct drug administration into the bladder show good survival rates, their therapeutic efficacy remains low, and nearly half of bladder tumors resurface within five years, requiring ongoing patient monitoring. “These treatments cause adverse side effects and have limited effectiveness, as evidenced by 5 yr recurrence rates of 30–70% and progression rates of 10–30%,” the authors wrote. Frequent hospital visits and the need for repeat treatments also contribute to making this type of cancer one of the most expensive to cure.
A promising alternative strategy involves the use of nanoparticles that are capable of delivering therapeutic agents directly to the tumor, and in particular nanoparticles that have capacity to self propel within the body.

Another crucial component for using nanobots for radionuclide therapy (RNT) is radioactive iodine, a radioisotope commonly used for the localized treatment of tumors.
Through their previous research the scientists had confirmed that this capacity for self-propulsion allowed the nanorobots to reach all areas of the bladder wall. This feature is advantageous when compared to the current procedure, as part of which, after treatment has been administered directly into the bladder, the patient must change position every half hour to ensure that the drug reaches all parts of the bladder wall.
This new study builds on initial results with the nanobots, to demonstrate not only the mobility of nanoparticles in the bladder, but also their specific accumulation in the tumor. The team were able to verify this using various techniques, including positron emission tomography (PET) imaging of the mice. “In vivo and ex vivo results demonstrated enhanced nanobot accumulation at the tumour site, with an eightfold increase revealed by positron emission tomography in vivo,” the authors noted. The team also reviews microscopy images of the tissues, captured using a fluorescence microscopy system developed specifically for this project at IRB Barcelona. The system scans the different layers of the bladder and provides a 3D reconstruction, thereby enabling observation of the entire organ.
The authors further explained, “Label-free optical contrast based on polarization-dependent scattered light-sheet microscopy of cleared bladders confirmed tumor penetration by nanobots ex vivo.”
Nanorobots lack specific antibodies to recognize the tumor, and tumor tissue is typically stiffer than healthy tissue. “However, we observed that these nanorobots can break down the extracellular matrix of the tumor by locally increasing the pH through a self-propelling chemical reaction,” continued IBEC researcher and study co-first author Meritxell Serra Casablancas, PhD. “This phenomenon favored greater tumor penetration and was beneficial in achieving preferential accumulation in the tumor.”
The scientists concluded that the nanorobots collided with the urothelium as if it were a wall, but in the tumor, which is spongier, they penetrated the tumor and accumulated inside. A key factor is the mobility of the nanobots, which increases the likelihood of reaching the tumor.

“… nanobots labelled even with low doses of 131I yield a net tumour volume reduction as their active motion leads to higher tumour accumulation. This efficacy at low doses should facilitate the translation of nanobots with therapeutic radionuclides to clinical settings.”
In addition, noted research co-lead Jordi Llop, PhD, a researcher at CIC biomaGUNE, “The localized administration of the nanorobots carrying the radioisotope reduces the probability of generating adverse effects, and the high accumulation in the tumour tissue favours the radiotherapeutic effect.” In their paper the investigators commented, “the motion-enhanced tumour accumulation enables radiotherapeutic self-propelled nanobots to have an effect at doses substantially lower than those required for efficient passive particles.” The next step for this, which is already under way, is to determine whether these tumours recur after treatment.
Commented Cristina Simó, PhD, co-first author, “The results of this study open the door to the use of other radioisotopes with a greater capacity to induce therapeutic effects but whose use is restricted when administered systemically.” As the authors concluded, “Overall, these results provide clear evidence of the potential use of urease-powered nanobots as efficient therapeutic carriers for bladder cancer therapy.”
The study consolidates the results of over three years of collaborative efforts between various institutions. The technology underlying these nanorobots, which Sánchez and his team have been developing for more than seven years, has recently been patented and serves as the foundation for Nanobots Therapeutics, a spin-off of IBEC and ICREA established in January 2023.
Founded by Sánchez, the company acts as a bridge between research and clinical application. “Securing robust funding for the spin-off is crucial to continue advancing this technology and, if all goes well, bring it to market and society,” Sánchez said. “In June, just five months after the creation of Nanobots Tx, we successfully closed the first round of funding, and we are enthusiastic about the future.”
Working with nanorobots has posed a significant scientific challenge with respect to the use of bioimaging techniques that can visualize these elements in tissues and the tumour itself. Common non-invasive clinical techniques, such as PET, lack the necessary resolution to locate these very small particles at a microscopic level. The Scientific Microscopy Platform at IRB Barcelona employed a microscopy technique using a sheet of laser light to illuminate samples, allowing the acquisition of 3D images through light scattering upon interaction with tissues and particles. Upon observation that the tumor itself scattered part of the light, generating interference, the scientists developed a new technique based on polarized light that cancels out all scattering from the tumor tissue and cells. This innovation enables the visualization and location of nanorobots without the need for prior tagging with molecular techniques.

![low-res Transmission electron microscopy image of the nanorobots. [Institute for Bioengineering of Catalonia (IBEC)]](https://www.genengnews.com/wp-content/uploads/2024/01/low-res.webp)
