Researchers from the University of Geneva (UNIGE) in Switzerland, in collaboration with the University of Tampere in Finland, say they have highlighted the critical role of paxillin, a protein that enables cells to perceive their environment and anchor at the right place with the help of cellular “crampons.” Without functional paxillin, the cell is unable to attach properly and slips continuously.
The team’s study “Structural and functional analysis of LIM domain-dependent recruitment of paxillin to αvβ3 integrin-positive focal adhesions,” published in Communications Biology, sheds new light on how cells adhere or migrate, mechanisms essential to the proper functioning of our organs, but also on how they can be involved in the development of metastatic tumors.
“The LIM domain-dependent localization of the adapter protein paxillin to β3 integrin-positive focal adhesions (FAs) is not mechanistically understood. Here, by combining molecular biology, photoactivation and FA-isolation experiments, we demonstrate specific contributions of each LIM domain of paxillin and reveal multiple paxillin interactions in adhesion-complexes,” write the investigators.
“Mutation of β3 integrin at a putative paxillin binding site (β3VE/YA) leads to rapidly inward-sliding FAs, correlating with actin retrograde flow and enhanced paxillin dissociation kinetics. Induced mechanical coupling of paxillin to β3VE/YA integrin arrests the FA-sliding, thereby disclosing an essential structural function of paxillin for the maturation of β3 integrin/talin clusters. Moreover, bimolecular fluorescence complementation unveils the spatial orientation of the paxillin LIM-array, juxtaposing the positive LIM4 to the plasma membrane and the β3 integrin-tail, while in vitro binding assays point to LIM1 and/or LIM2 interaction with talin-head domain.
“These data provide structural insights into the molecular organization of β3 integrin-FAs.”
To ensure our survival, each cell performs specific functions in coordination with their neighbors. In such a dynamic system, the migration of cells and their anchoring at the right place are essential. But how do cells manage to coordinate with each other? Scientists long believed that cells communicate mainly through chemical signals, such as hormones. However, recent discoveries suggest that mechanical signals play a major role in cell coordination.
“This is why we started to study the ability of cells to decipher and respond to their physical environment,” explains Bernhard Wehrle-Haller, PhD, professor in the department of cell physiology and metabolism at the UNIGE Faculty of Medicine. “Especially as it could help us to understand how cancer cells use these mechanisms to invade other organs and form metastases.”
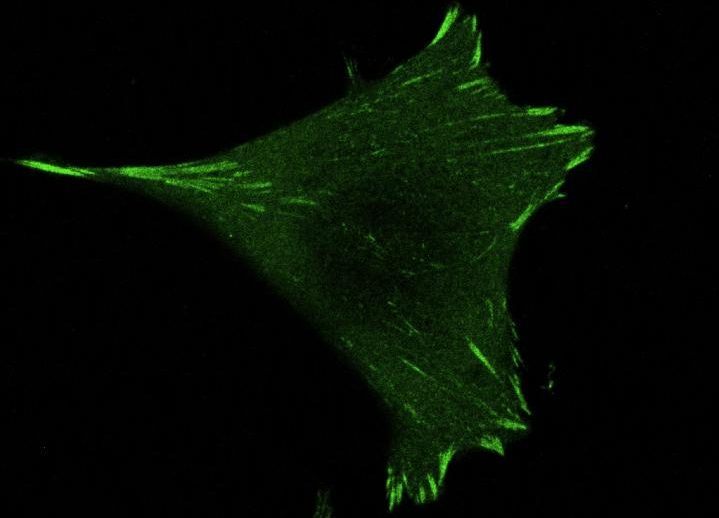
When a cell has to move, it “senses” its environment with the help of proteins (integrins) on its surface. When the cell detects a suitable location, a complex network of proteins (focal adhesions) is then set up to form cellular crampons that anchor the cell to its environment. “But how is this anchoring mechanism regulated? This is what we wanted to find out,” explains Marta Ripamonti, a researcher in the Wehrle-Haller lab and first author of the study.
By studying paxillin, one of the many proteins that make up these crampons, researchers were able to unravel the mystery, according to Wehrle-Haller. “We knew that this protein played a role in the assembly of focal adhesions, but we didn’t expect it to be the key regulator.”
Without functional paxillin, cells are unable to anchor, regardless of the suitability of their environment. In addition, paxillin also “informs” the cell that anchoring has taken place correctly, thus transforming a mechanical response into a biological signal that the cell can understand.
These in vitro experiments highlight the major role of paxillin in the migration and adhesion of healthy cells, but they could also be a starting point for a better understanding of cancer development.
“It is indeed likely that cancer cells use paxillin to find a place that enhance their survival. Would it be possible to block this mechanism in tumor cells and prevent the formation of metastases? We think so,” says Wehrle-Haller.