Cancer is the Houdini of diseases because it escapes elaborately contrived predicaments. Like Houdini, who was known to emerge triumphant after being handcuffed, locked in a trunk, and tossed into a river, cancer is known to progress after being subjected to combination therapies, never mind single therapies. Even the best therapies fail to work consistently across all patients.
To ruin cancer’s career as an escape artist, developers are working on new drugs, including more effective inhibitors. For example, Effector Therapeutics is working on selective translation regulator inhibitors (STRIs). According to the company, STRIs represent a radical approach, one that focuses on the underexplored potential of targeting eukaryotic initiation factor 4F (eIF4F), a protein complex that helps start the translation of many proteins. Basically, STRIs target eIF4F elements, blocking the expression of multiple oncoproteins without also inhibiting expression of proteins that cells need for healthy functioning.
Seeing opportunity in eIF4F
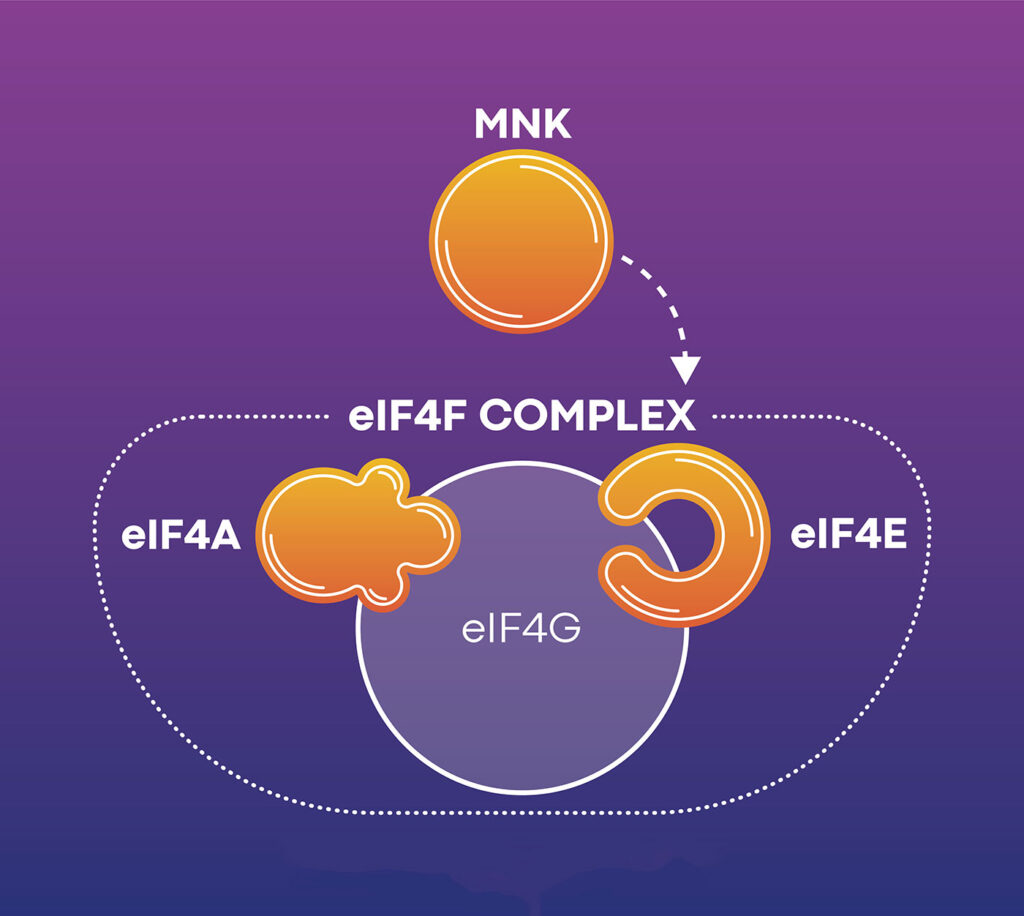
The eIF4F complex mediates the recruitment of ribosomes to mRNA, including some of the most difficult-to-translate mRNAs. Overall, the mRNAs acted upon by eIF4F account for about 5% of the proteins in a cell.
“Some messages don’t get translated into proteins unless eIF4F is activated,” says Steve Worland, PhD, co-founder, president, and CEO of Effector Therapeutics. And some of the messages that are acted on by eIF4F are translated into proteins that profoundly change the behavior of the cell. “For example, they may drive the cell cycle, protein synthesis, or DNA replication and separation,” Worland details. “The problem is that in cancer, these proteins, which should only be made infrequently, are being made all the time.”
Inhibiting production of the oncoproteins made via the eIF4F complex causes the cancer cells to stop dividing and, often, to die. “It’s not cytotoxic per se,” Worland notes, “but it restores the cell’s ability to undergo apoptosis.”
Advancing the clinical pipeline
Effector Therapeutics has two drugs in Phase II trials: tomivosertib (“Tomi”) and zotatifin (“Zota”). Tomi stimulates the immune system and blocks immunosuppressive proteins, thus allowing the immune system to attack cancer cells. (“The immune cells,” Worland points out, “use the eIF4F complex to upregulate immunosuppressive proteins in cancers.”) Zota, in contrast, works inside tumor cells, interfering with cell cycle progression by blocking cyclin-dependent kinases and, Worland emphasizes, a “tiny subunit” of the eIF4F complex.
In one trial, Tomi was administered to 39 patients who had already received a checkpoint inhibitor that failed to produce a sufficient response. “When we administered Tomi to patients who already were failing checkpoint inhibitor therapy, we could restore checkpoint inhibitor activity,” Worland reports. “Tumors that were growing would often stop growing quite quickly.” In some of the patients with lung cancer, progression was halted for an average of one year.
In another trial, Zota was administered to 19 women with late-stage breast cancer who had completed an average of four lines of prior therapy. There was a 26% partial response rate. Effector Therapeutics is currently conducting dose-escalating studies with Zota as a second line, combination therapy. According to Worland, the company should have data from both programs by the end of the year.
Defying conventional wisdom
Scientists have tended to ignore the targeting of translation regulation as a potential therapeutic approach. “We were all taught that translation is a housekeeping function,” Worland explains. “That’s true for 95% of proteins, so people missed this regulation component.”
The other hurdle was overcoming the extraordinarily difficult chemistry surrounding proteins that interact with RNA and RNA-binding proteins. “It is notoriously difficult to develop a small molecule with the right properties to be a drug for human treatment that blocks this complex that interacts with RNA,” Worland says. “There are published papers saying that these are great targets, but that no one will ever crack them.”
Worland asserts that before Effector Therapeutics was founded, no one had shown that you could get selectivity with a small-molecule drug inhibitor. So, one concern was that that targeting eIF4F would act universally. But according to Worland, the company’s results have been “quite good.” He adds, “We still have to understand the full safety profile and dose hundreds of patients. We’re not there yet.”
Offering multiple lines of attack
Worland co-founded Effector Therapeutics in 2012 with Kevan Shokat, PhD, and Davide Ruggero, PhD, both researchers at the University of California, San Francisco. The company went public in 2021 by merging with Locust Walk Acquisition Company, a special purpose acquisition company.
Worland came to cancer research from the antiviral drug development world, where he occupied high-ranking positions at Anadys Pharmaceuticals, Pfizer, and Agouron Pharmaceuticals. His experiences impressed on him the need to anticipate and counter viral mutations and resistance—as well as the need for countermeasures offering multiple lines of attack.
“I was looking for an oncology technology that would provide a combination therapy to hit different factors at once,” Worland recalls. “[It] would also look like a targeted therapy.” In his estimation, a targeted combination therapy would “improve the outcome for the majority of cancer patients.”
“I went around the country to talk with researchers at the top universities about their research,” Worland continues. “I was lucky enough to meet Kevan and Davide. When we were talking about kinase inhibitors, Kevan started explaining translational regulation. Collectively, we saw this as a way to address cancer’s complexity.”
Demonstrating (and inspiring) commitment
“We have a deep commitment to structure-based drug design using atomic information,” Worland says. “I’ve worked at bigger companies, and undoubtedly we would have dropped these programs and moved on to something else.” He adds that the company’s persistence yielded encouraging results: “We saw that we had a small molecule that blocks eIF4F and that knocks down the proteins (specifically, CDK4) that matter for cancer cells. That gave us the conviction that we were on to something.”
Enthusiasm over Effector Therapeutics’ results needed to extend beyond the company. “To do pioneering science and work on novel biology inside a commercial entity, you have to spend a lot of time with investors and potential partners explaining what you’re doing,” Worland remarks. To ensure an adequate cash flow, the company raised $16 million in registered direct offerings recently, extending its runway into Q2 2024. The funding will also help the company to continue de-risking its assets and to launch dose-escalation studies before approaching the FDA. The company’s earliest program, which is focused on an eIF4F subunit called eIF4E, is in preclinical studies with Pfizer.
When Effector Therapeutics began, capital was tight. “The economy was just coming out the Great Recession,” Worland recalls, “and many venture capital firms had gone out of business.” Now the economy is tightening again. But Worland remains optimistic. “This time,” he declares, “we’re a clinical company.” The implication is that Effector Therapeutics can give presentations that highlight clinical data and tangible patient results rather than details about the company’s science and its potential.
“The challenge now is to continue to show how this works for patients, and to position our therapies,” Worland says. That entails showing product differentiation as well as expanding indications for which this approach may be effective.


