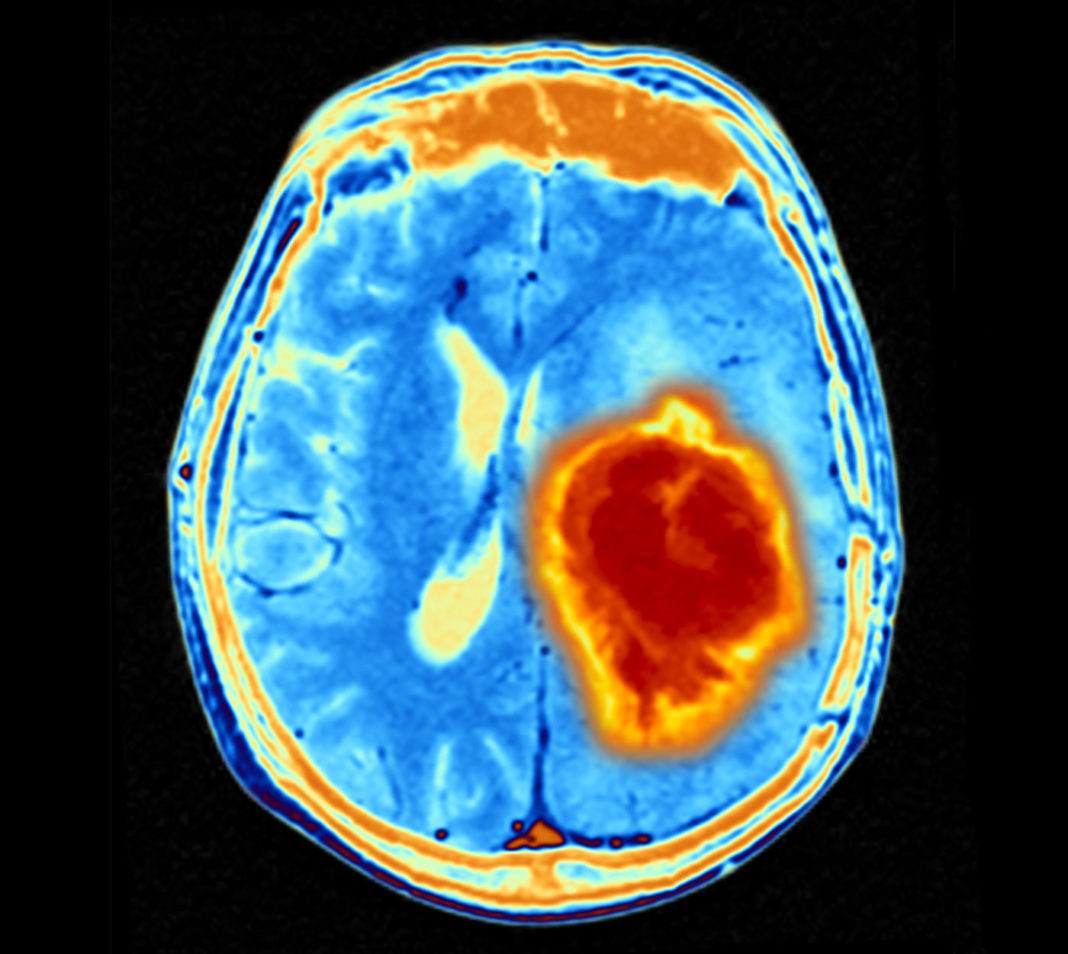
Glioblastoma (GBM) is the most aggressive and lethal form of brain tumor. Yet, the underlying molecular mechanisms driving GBM infiltration are not fully understood. Now, researchers from Baylor College of Medicine working in animal models have uncovered a novel process by which neurons in locations remote to the primary tumor provoke expression of genes from glioblastoma that subsequently drive tumor infiltration.
The findings are published in Nature in an article titled, “Remote neuronal activity drives glioma progression through SEMA4F.”
“The tumor microenvironment plays an essential role in malignancy, and neurons have emerged as a key component of the tumor microenvironment that promotes tumorigenesis across a host of cancers,” wrote the researchers. “Recent studies on GBM highlight bidirectional signaling between tumors and neurons that propagates a vicious cycle of proliferation, synaptic integration, and brain hyperactivity; however, the identity of neuronal subtypes and tumor subpopulations driving this phenomenon is incompletely understood. Here we show that callosal projection neurons located in the hemisphere contralateral to primary GBM tumors promote progression and widespread infiltration.”
“Previous studies have shown associations between the presence of GBM and increased neuronal activity in surrounding brain regions, which can promote tumor progression,” said first author Emmet Huang-Hobbs, PhD, who was a graduate student in the lab of Benjamin Deneen, PhD.
To study how neurons stimulate GBM infiltration, the researchers first determined which neuronal populations promoted glioma intrusion.
“Severing the corpus callosum eliminated the neuronal activity-dependent acceleration of GBM infiltration that was observed with the intact control, supporting that an intact corpus callosum is necessary to promote glioma progression and implicating CPNs’ long-range projections in remotely driving GBM infiltration,” Huang-Hobbs said.
“The findings suggest that GBMs receive neuronal inputs from a host of brain regions, implying that exposure to a diverse range of neuroactive compounds can potentially influence tumor growth. It’s now clear that tumor-neuron interactions are more widespread than previously thought,” said Deneen, professor and Russell J. and Marian K. Blattner chair in the department of neurosurgery, director of the Center for Cancer Neuroscience, and a member of the Dan L. Duncan Comprehensive Cancer Center at Baylor. He is also the corresponding author of the work.
“In collaboration with the labs of Baylor researchers Jeffrey L. Noebels and Ganesh Rao, we found evidence suggesting that GBM and CPNs have a two-way conversation,” Huang-Hobbs said. “CPNs promote tumor infiltration, and the tumor affects neuronal connections or synapses. The tumor remodels local neuronal synapses and makes direct synaptic connections, raising the possibility that it alters brain circuit activity in these regions that are distant from the primary tumor.”
Further analyses showed mechanistic details underlying these observations. The researchers found that the infiltrating tumor population is enriched for axon guidance genes, including SEMA4F, which they identified as an essential factor for glioma progression and neuronal activity-dependent infiltration. Interestingly, SEMA4F also promotes neuronal hyperactivity.
The study demonstrates that subsets of neurons in locations remote to primary GBM promote malignant progression, and also show new mechanisms of glioma progression that are regulated by neuronal activity.